Chara Biologics and The Joy of Ketamine
If you’re an avid follower of this blog, you know that as I perform deep dives into various players in the stem cell wild west, the strange stuff that can be found is often astounding. Today I’ll take another look at Chara Biologics that will begin with a strange clinical trial and end with the company founder tripping on Ketamine on YouTube. As I often say, you can’t make this stuff up.
What is Chara Biologics?
Chara is Greek for “Joy” which is important, as the founder of this birth tissue distributor’s name is “Joy Kong”. Joy is a west coast psychiatrist who founded this birth tissue distributor several years back. That’s an important distinction because Chara Biologics, according to past and current FDA tissue registrations is a packager and distributor of birth tissue products and NOT a manufacturer. Meaning that Chara private labels someone else’s product.
Chara sells both amniotic and umbilical cord products. If you want to see how Chara views itself, look no further than it’s website:
Wow, IMHO that’s a risky statement that Chara considers itself medicine. Why? All Chara has is a quick 45-minute FDA tissue registration without any clearance or approval from the FDA. Hence, calling yourself a drug requires another pathway for actual FDA review involving tens to hundreds of millions of dollars and often multiple clinical trials PER CLINICAL INDICATION.
Issues with the FDA
One of the reasons I was surprised to see Chara calling itself a drug is that the company has already been warned by the agency for making claims it can’t back up (see letter above with link). Basically, the FDA determines how to regulate a product based on its claims and in this case because Chara is someone else’s tissue. Also, that regulation hinges on how much processing is required for Chara’s product.
Chara’s claims of its CharaCrore product having live stem cells and its ability to be used to treat various incurable diseases makes that product a drug without any approval or clearance from the FDA. The FDA requested that Chara stop making these claims and/or pursue FDA clinical trials. How did Chara respond? More on that below.
Chara’s Claims of Live Stem Cell Product
First, both our lab and the Translational Medicine Institute at CSU tested Chara’s claim that its CharaCore product had copious amounts of live and functional mesenchymal stem cells. That data is below:
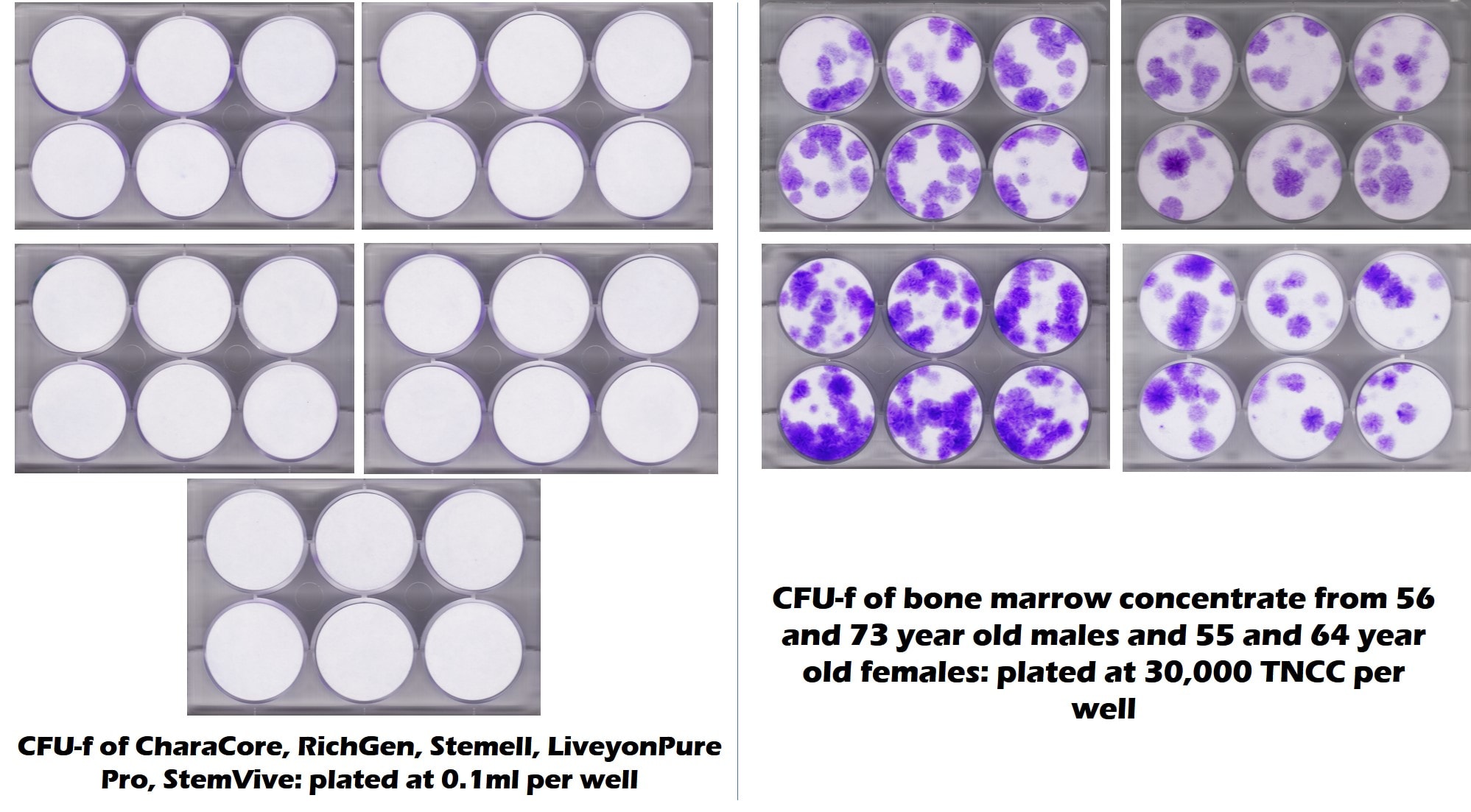
The all-white in the 6-well plate in the upper left-hand corner clearly means that CharaCore has no mesenchymal stem cells (MSCs) in this common CFU-f test, which would show up as purple dots. The middle-aged and elderly bone marrow to the right has purple dots, hence stem cells. So any claims on Chara’s part that it sells a product containing MSCs were not verified by independent tests at two separate labs.
The Clinical Trial
So what do you do when the FDA says you need clinical trials AND THEN it’s review and approval BEFORE you can sell your product? You back off the claims and pull your product from the market and meet with the FDA to begin a clinical trial. What did Chara do?
In the past several months, a new banner appeared on the Chara Biologics website:
The orange banner on the bottom here states that there is a clinical study for traumatic brain injury that uses a Chara Biologics product. We tried several times to get any information on this study, but only recently did the company respond. This is what we were able to find out:
- This is not a free study, in fact, the total cost for the patient is $15,000
- The study lasts for 6-months
- It’s three IV infusions spaced one month apart.
- Each infusion includes Dr. Kong’s own patented “stem cells” at 30 million
- The infusion is made from placental derived-umbilical cord tissue, umbilical cord blood, and amniotic membrane
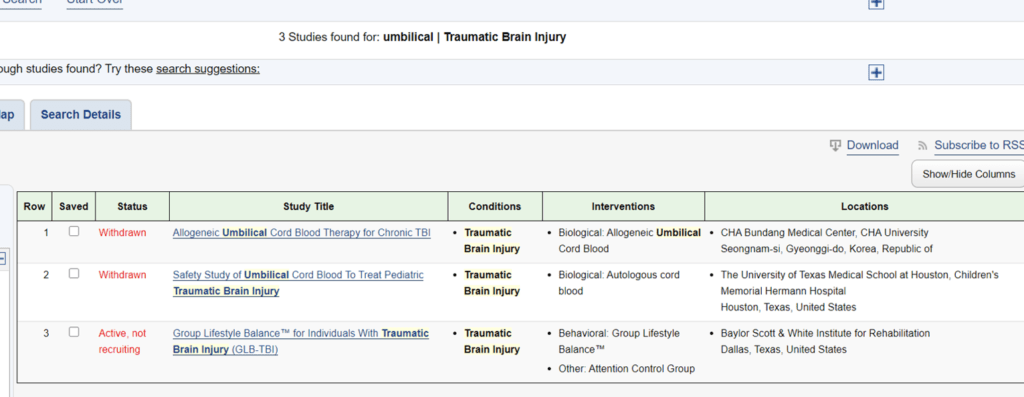
So is this an FDA-approved study authorized through an IND? If it were, it would be required to be listed on Clinicaltrials.gov. I searched that site and this is what I found:
Nothing. Under the topic of traumatic brain injury, no Chara Biologics study was found. In fact, of the three studies listed that had nothing to do with Chara, two were withdrawn and the other has nothing to do with stem cells. Also, no Chara study is listed at Clinicaltrials.gov under any topic. So whatever this study is or isn’t, it isn’t an FDA approved and monitored study.
Why is it important that this isn’t an FDA approved study? Because Chara is still claiming that patients will be treated with stem cells. That alone requires FDA oversight of the research.
Chara Biologics is Still Proudly Claiming that it is Producing Stem Cells
The Chara Biologics website has changed it’s marketing message since the FDA letter. Now instead of claims of live stem cells, we see this:
“CharaCore ™
A high potency, minimally manipulated placental-derived tissue transplant product. Help assist the body to repair and regenerate. Even though this product contains live cells, it does not rely on live cells or the metabolic activities of live cells for its therapeutic benefits.”
Given that scientifically, nobody on earth knows how any umbilical cord product works, IMHO Chara’s claim that its product has live cells and that its product doesn’t work by the metabolic activity of those cells is ridiculous.
We also still see this on the website:
“Why is Birth Tissue a SUPERIOR source of stem cells? Because the cells are young and more therapeutically active”
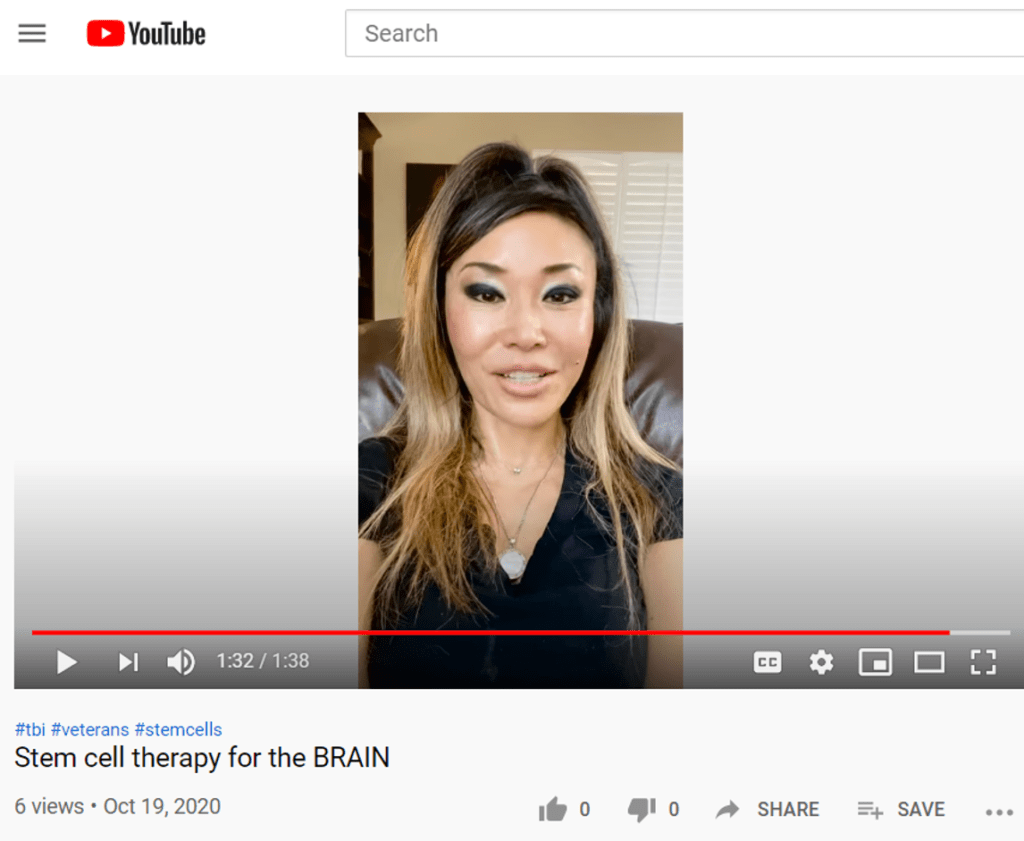
So Dr. Kong seems to be doubling down on the idea that her products contain stem cells by trying to be clever in how things are worded, but IMHO there are still loads of FDA violations here. For example, look no further than Joy Kong’s YouTube channel to see that she is still actively hawking stem cells. In the below video she claims that Chara’s “stem cells” are being used in the above-mentioned study. She also states that CharaCore is “The most potent and comprehensive stem cell product on the market”.
This wasn’t posted last year BEFORE the FDA letter, it was posted about a week ago.
Or how about this from a Chara patient email:
“When Compared to Other Neonatal Allograft Stem Cell Sources: Chara products are all highly rich in MSC’s, with 20-30% of the total cell populations being MSC’s.”
What percentage of the CharaCore product was live and functional MSCs based on the above tests? 0%.
Ketamine Infusion?
As I have documented in the past, IMHO Chara Biologics has problems with its integrity and honesty. First, you would think from the claims of being a revolutionary future medicine that it would be manufacturing that product. Nope, it just private labels someone else’s product. Second, the company clearly has FDA regulatory issues with the agency calling out its claims. Third, Chara’s new study requires FDA oversight and apparently has none. Finally, despite being told by the FDA that Chara can’t make claims that it’s selling mesenchymal stem cells (which don’t exist according to two independent lab tests), Chara continues to claim that it’s selling stem cells. However, it gets far weirder than that…
A few months back I got an anonymous email from what could have been a disgruntled ex Chara employee or sales rep. While there were certain claims made about Joy Kong, the company’s founder, most couldn’t be independently verified. However, one that could, which blew me away, was the below video which was publically available on Joy Kong’s YouTube channel. The video literally shows Joy Kong, M.D. tripping on Ketamine:
All I can say is, “Yikes!” It’s one thing to believe in the potential for drugs like Ketamine in psychotherapy, but quite another for a physician to post a video using that drug. From the video we learn:
- Joy had six Ketamine infusions to make the results of this treatment last longer
- These six sessions were the best six hours of her life
- She has never had such beautiful and powerful experiences
- The ketamine took her to a different dimension
- She felt like she touched the hand of God
- The journey took her to a place where she felt the breath and pulsation of the universe
- Joy considers our world (compared to the one she visited during the Ketamine infusion) monochrome
There’s just not too much more I can say about a physician owner of a “stem cell” company putting up a public video of tripping on a psychoactive drug.
The upshot? Chara Biologics is still claiming to sell stem cells, despite their FDA warning. They have started some sort of pay for play study that doesn’t have FDA approval nor is it registered at Clinicaltrials.gov. Perhaps most astounding is that the founder of the company, a licensed medical doctor, is tripping on YouTube after her 6th Ketamine infusion! You can’t make this stuff up!

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.