New Amniotic “Stem Cell” Review Paper: Misinformed or Misleading?
One of the things that’s often hard for patients to understand is that when it comes to using stem cells for orthopedic conditions, the universities are way behind private-practice physicians. The good news is that we’re seeing some universities, like Stanford, Emory, and the Mayo Clinic, begin to embrace simple cell therapies. The bad news is that other universities are still way behind the average private-practice doctor in using these therapies. An example of that issue is a recently published amniotic stem cell review paper by physicians at Rush University in Chicago. These guys are likely well meaning, but they clearly have little knowledge of what’s not in commercially available amniotic tissues being sold on the market today.
What Are Amniotic Tissues?
While I’ve blogged on this topic many times, amniotic tissues are derived from the waste products of the delivery of a child—namely, the amniotic fluid that surrounds the baby as well as the birth sac and placenta. Given that these tissues are cheap and plentiful, they’ve been sold for many years for use in wound healing, neurosurgery, and ophthalmology. These same amniotic products have been more recently used in orthopedic injuries, despite a lack of data showing that they could help these problems. More concerning is that many physicians have been convinced by sales reps that commercially available amniotic tissues contain stem cells, but they, in fact, contain no live cells. Even more concerning is that many physicians extend this ruse to their patients, convincing them to spend big bucks on amniotic “stem cell” injections that, in fact, contain no stem cells.
Conflicts?
Before I read many papers, I always do a little snooping on the authors, just to see who they are, who they work for, and whether there may be bias in what they write. In this paper, they list 14 different companies that have funded research, paid royalties or speaking or consulting fees, or provided stock or stock options. While this isn’t automatically a significant conflict, three of the companies (NuTech, Arthrex, and Zimmer) make or vend amniotic tissues. Hence, the authors have conflicts of interest on this topic. Having said that, I run a large, international, medical-group practice that has chosen not to use amniotic stem cells, so some would say that I have the opposite bias.
The Familiar Amniotic Stem Cell Shuffle Is Alive and Well
Here’s my big concern with this paper. While I expect the usual rank-and-file uneducated physician to make the mistake that commercial amniotic products contain living stem cells, I don’t expect university physicians to make that same mistake.
The problems in the review paper begin when the authors use this paragraph heading in the paper:
“AMNIOTIC MEMBRANES AS A SOURCE OF STEM CELLS”
While there is truth in this statement (i.e., amniotic tissues when they are living do contain a small population of stem cells), my antenna went up, as the paper is about using these tissues in sports medicine, where specifically no physician will be using live amniotic tissues but instead the nonliving tissues sold to doctors. To 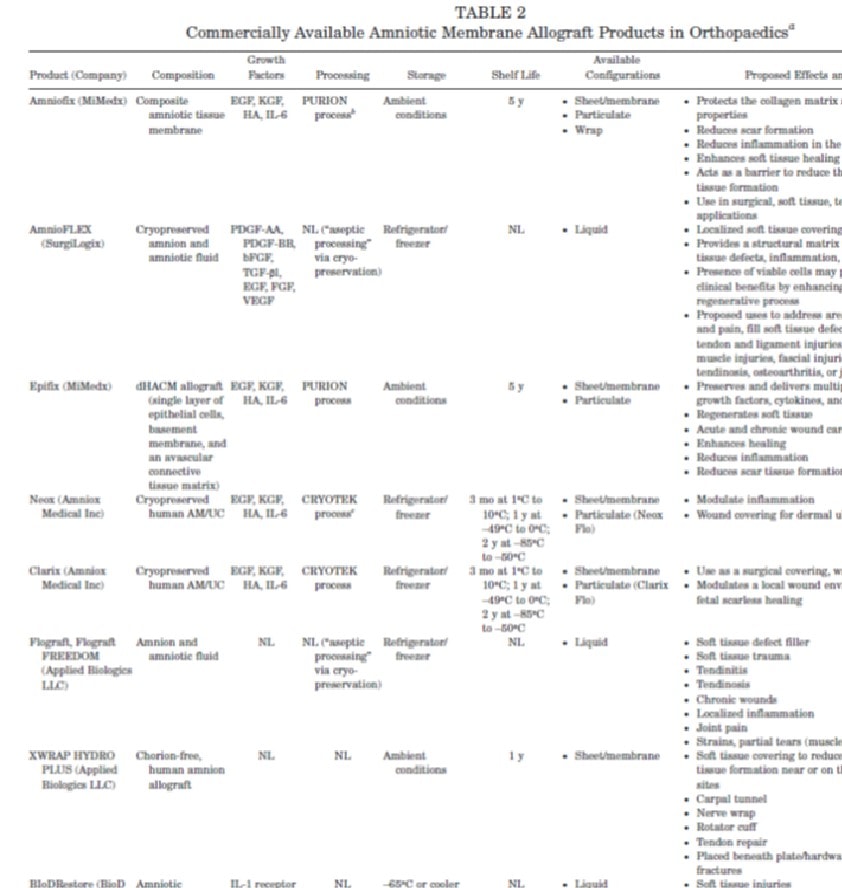
As I read on in the paper, the paragraph above didn’t explain the difference between live tissues and amniotic tissue products. The next paragraph just describes the different stem cell types, how they are isolated from amniotic tissues, and how they can differentiate into orthopedic tissues. Again no disclaimer. Finally, a section emerged that would surely contain the explanation:
“COMMERCIALLY AVAILABLE HUMAN AMNIOTIC MEMBRANE–DERIVED PRODUCTS”
However, this section was a disappointment, as while it explains that these commercially available tissues are processed differently and discusses the “known effects of AM processing on its mechanical, biological, and cellular properties,” nothing is said to make it clear to physicians that when they purchase and use these products, they are not using a “stem cell” product. Even more disappointing is this statement later in the paper, which only adds to the suggestion that there are stem cells in these products: “Three major strategies have been employed in these early studies: (1) using AM as a scaffold for bone marrow-derived mesenchymal stromal cell (MSCs), (2) using AM as a scaffold for delivery of chondrocytes, and (3) inducing AM-derived pluripotent cells toward a chondrogenic phenotype.” This last statement would make any reader believe that by using commercial amniotic tissues they are using an amniotic stem cell product.
While the authors up to this point in the paper don’t come out and directly state that commercially available amniotic tissues contain stem cells, they have come pretty close. I thought that would be the end of their flirting with the issue until I came across this statement: “In summary, AM offers promise as an alternative to collagen I/III membrane scaffolds for 2-stage cartilage repair and as a source of pluripotent cells that does not require a morbid harvest (such as a bone marrow aspirate or a cartilage biopsy specimen).” Yikes, they finally dropped the big one! They continue to insert both feet into their collective mouths with this additional statement: “Amniotic membrane–derived products have the advantage of minimizing the ethical issues shared by embryonic stem cells while still having the promise of an easily attainable population of pluripotent cells…”
I was pretty dumbfounded by this amniotic tissue review paper. As I said, I expect private-practice physicians to make this novice mistake, but we all hold academic physicians to a higher standard. Having said that, most academic centers are way behind private practitioners when it comes to the deployment of regenerative orthopedic therapies, so maybe it’s not too surprising that these doctors don’t know that these tissues don’t contain live and viable stem cells.
The upshot? I’m hoping these academic surgeons just don’t know what they don’t know and like many other physicians they bought the hype generated by sales reps. Hopefully, this review of their review will prevent them from making this same mistake in future papers. In addition, while we’ve done the research to vet the claims of sales reps claiming that commercially available amniotic tissues don’t have live stem cells, I welcome any research these surgeons publish to the contrary.
If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.