Embryonic vs. Adult Stem Cells
Embryonic vs Adult Stem Cell Research
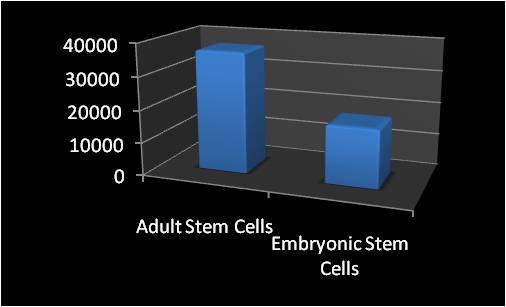
We’ve all heard about embryonic stem cells (ESC’s). They have been in the news and the focus of controversy for a decade. We’ve seen far less media coverage of adult stem cells. Adult stem cells (ASC’s) are found in everyone’s body and they are capable of repairing a host of tissues. In fact, the research on adult cell lines has surpassed embryonic. Above is the result of a search I did yesterday in the National Library of Medicine, looking at all embryonic stem cell published papers vs. the total on all adult stem cell lines. As you can see, for every one ESC paper, there were at least two ASC papers. In addition, the research on ASC’s is vastly more mature, focusing on animal models of tissue healing, dosing, early human cases, medication interaction with cells, possible complications, etc… The ESC research is more test tube and review articles on what might be possible someday. Looking in specific categories, I found the following:
· Cartilage Repair: 230 articles on embryonic vs. 1,113 for just one adult stem cell line (mesenchymal stem cells)
· Myocardial Infarction: 186 for embryonic stem cells vs. 341 for adult mesenchymal stem cells, 69 for endothelial progenitor cells
· Wound Healing: 114 for embryonic stem cells vs. 330 for adult mesenchymal stem cells, 565 for adult epithelial stem cells
For a more detailed analysis, take a look at the American Stem Cell Therapy Assocition (ASCTA) white paper on adult stem cells vs. embronic stem cell research. Summary? Adult stem cell research is much farther ahead. In addition, as I have posted on before, if the cells are autologous (from the same patient), they also have a much lower risk profile. While one day embryonic cells may rule the day, for now, adult stem cells are beating the pants off ESC’s in practical research.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.