New Research On Amniotic Injections: ReNu

Credit: Shutterstock
As I have maintained for years, amniotic tissue may have a role in musculoskeletal treatment, but early sales scams hurt the industry. In the meantime, several companies have begun to pay for clinical trials to see where this stuff may work the best. Today we’ll cover some new data published on a product named ReNu.
Amnio Craziness
If you read this blog, you can’t ignore all of the scams surrounding amniotic tissue. From claiming that these products had stem cells to claims of crazy growth factor concentrations to recent Medicare scams, this field has been a mess. Having said that, the idea that these tissues may help patients with orthopedic problems is not far-fetched. I have colleagues who have used these products for certain applications like tendon healing that have seen good results. I have used them in combination with PRP to treat rotator cuff tears and have seen good results. So at some level, I’m a believer that there’s something there.
Real Amnio Research
Companies that are serious in this space have been conducting randomized controlled trials to see what this product can do. Organogenesis, the company that makes an amniotic suspension has been funding this type of research for several years. In fact, the company received an FDA RMAT (a type of fast-track) in January of this year. Regardless of the ultimate results of that application, I want to congratulate the company for spending that money and doing the research.
Before I began this review of the existing published ReNU research, I had only briefly scanned their first paper and added it to a blog. Hence, I began with the concept that amniotic tissue could help knee OA based on what the study abstract said. However, this week, another company reported poor clinical trial results in this same space, so that meant that taking a closer look at the ReNu data was a good idea.
ReNu Knee OA Study #1
The first study on ReNu was published in November of 2019 (1). 200 subjects with moderate knee osteoarthritis were randomized to receive ReNu, hyaluronic acid, or normal saline injections. This first study only went to 6 months, so these groups were compared at time 0, 3 months, and the last data point was at 180 days. So how did ReNu do? When I took the liberty of graphing the data points myself, the problem was that is was “squirrelly”.
Why use that term? What I mean is that when I looked at the three groups for the questionnaires they used (EQ-5D-5L, KOOS, SANE, Tenger, and VAS), whenever it looked like ReNu was winning, one of the other groups would have outcome scores that beat or matched it. Let’s look at one of those scores to illustrate the point. It’s called SANE, which stands for Single Assessment Numeric Evaluation. It’s one number between 0-100 that represents the overall percentage improvement:
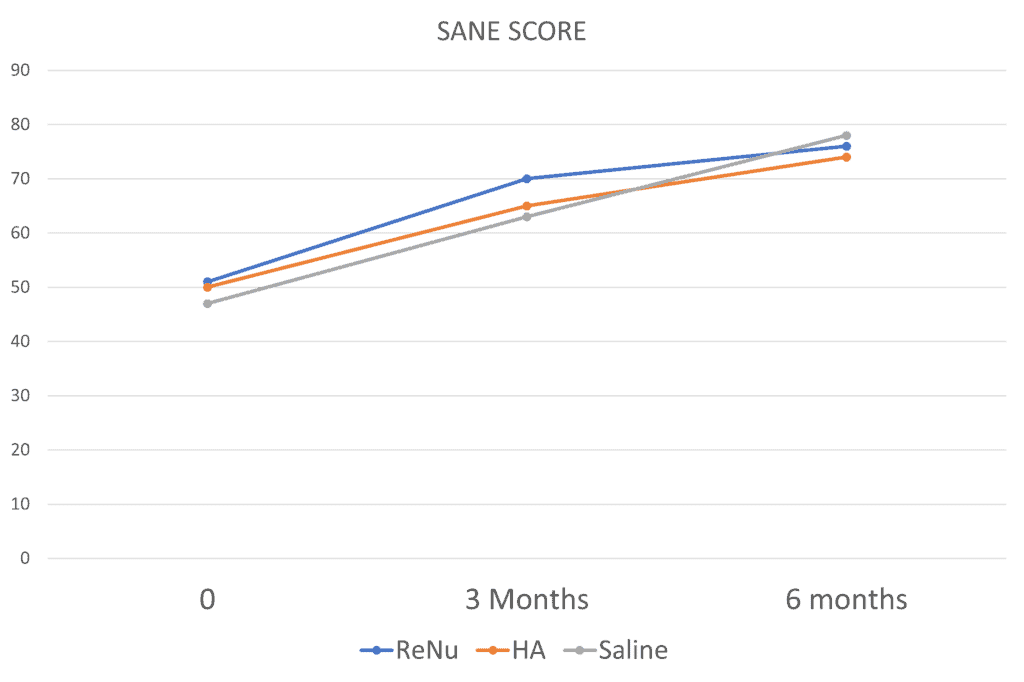
At three months it looks like ReNu is winning, but not by much. At 6 months, there is no real clinical difference here between ReNu and the two control groups (HA and saline). How about the KOOS? That’s a knee functional questionnaire. Here I graphed the Sports and Recreation domain from that questionnaire:
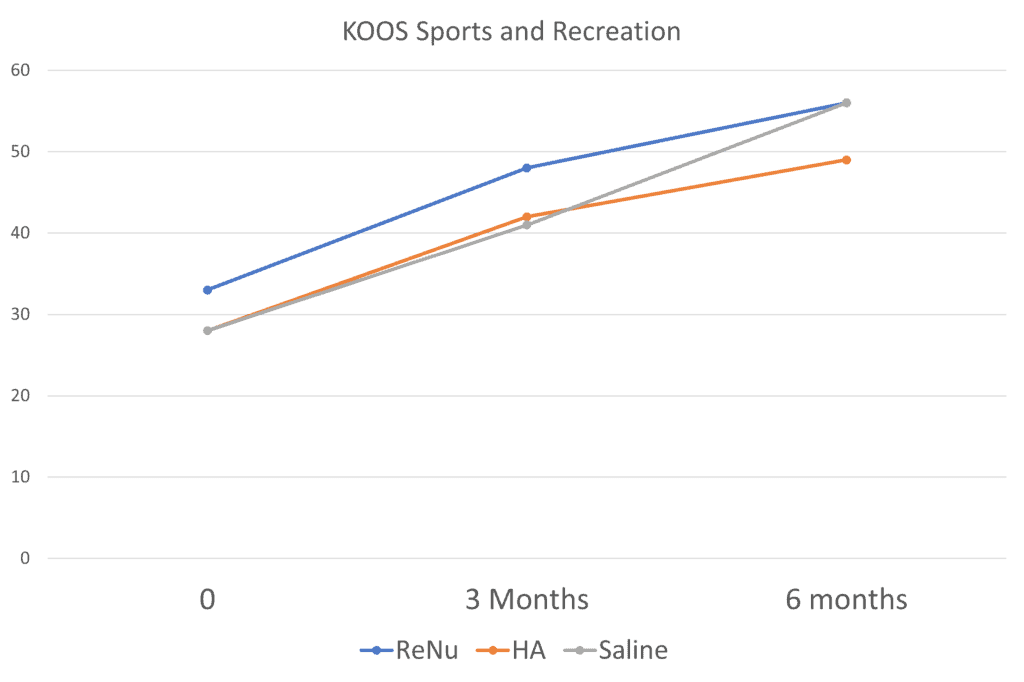
Here the saline group matched ReNu at 6 months. It’s much the same with many of the other scales and subscores. Just when you think ReNu is going to rock it, it doesn’t. So how did they get to claim in the study abstract I read that ReNu was better than HA or saline? By picking and choosing which subscales were statistically different at specific time points. For example, the authors claimed that the KOOS pain domain score was better for ReNu:
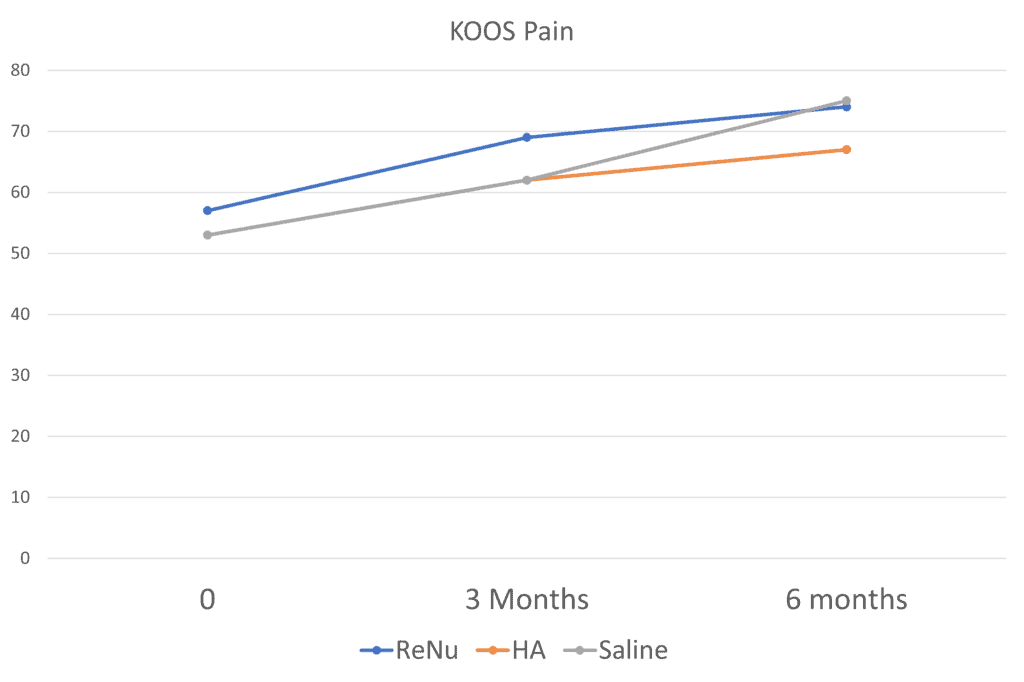
Sure enough, at 3 months, ReNu worked better (the blue line is the highest). However, saline overtakes ReNu at 6 months. That’s what the rest of the data analysis is like. In major subscales and questionnaires, the effects of ReNu look better until they don’t or ReNu beats HA and then gets creamed by saline. Or it beats saline and then gets matched by HA.
What Does a PRP Trial Look Like?
At the end of the day, ReNu and other amniotic products are competing in the marketplace with PRP. In order for the much higher price of these products to be justified, they need either to match that efficacy or beat it in similar patients with knee osteoarthritis. If they match the efficacy, then they may be successful because they’re “off the shelf” without the need to draw blood, but only if the final price of the product is somewhat close to PRP.
So what do the KOOS graphs look like for a study that tested PRP vs a steroid injection in patients who in general had much more severe knee arthritis (meaning here the PRP should be performing more poorly than Renu did in its study on patients with only moderate disease)? Let’s take a look at the same graphs from a study by Jubert et al (2):
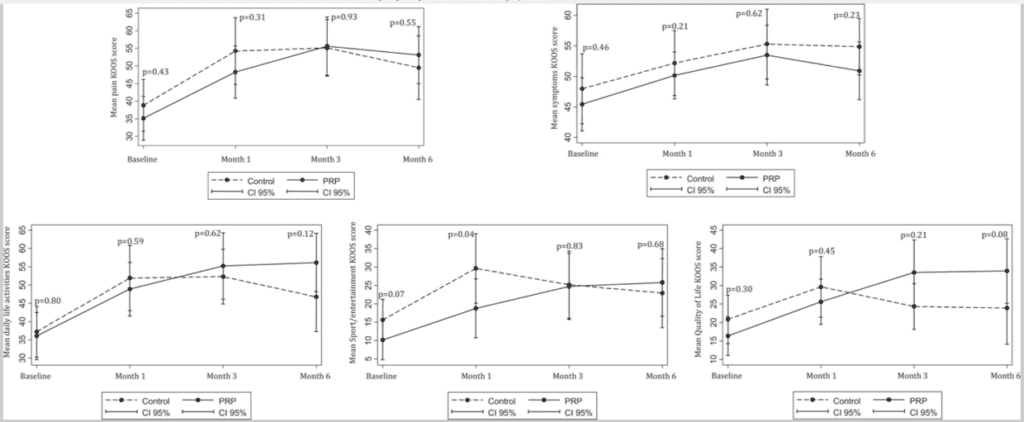
Here we see a far better separation between the PRP and the steroid groups in more subscales than we saw in the ReNu study. Again, this was in patients with more severe disease than those tested in the ReNu trial.
Responder Analysis?
One of the games often played when the data in a randomized controlled trial doesn’t show a clear separation between the groups is what’s called a responder analysis. This involves re-swizzling the data such that you look not at the average responses in each group, but the percentages in each group who met a threshold response. The good news is that this is a bit more clinically relevant, but the bad news is that it’s often being done because the group comparison data came back weaker than expected. Hence that’s what the authors of the 2019 study do and that makes ReNu look better when compared to saline and HA.
The HUGE Problem with the ReNu Study
Forget about barely-there results when the groups are compared. Forget about responder analysis. There’s a more glaring problem with this study that when I first found it, I had to call one of our research experts to confirm because I couldn’t believe what I was reading. I’ll call it “fatal drop-out”.
One of the hardest things to do in a randomized controlled trial is to keep people in their groups for the duration of the study. In a good RCT, you may lose a handful of subjects by the end, but hopefully not more than 10-20%. If you lose more than that, these are patients that are an unknown. You don’t really know how they responded to the treatment they received, which dramatically impacts the veracity of the result.
So how many people dropped out of which groups in the ReNu study? This is the key figure from the study:
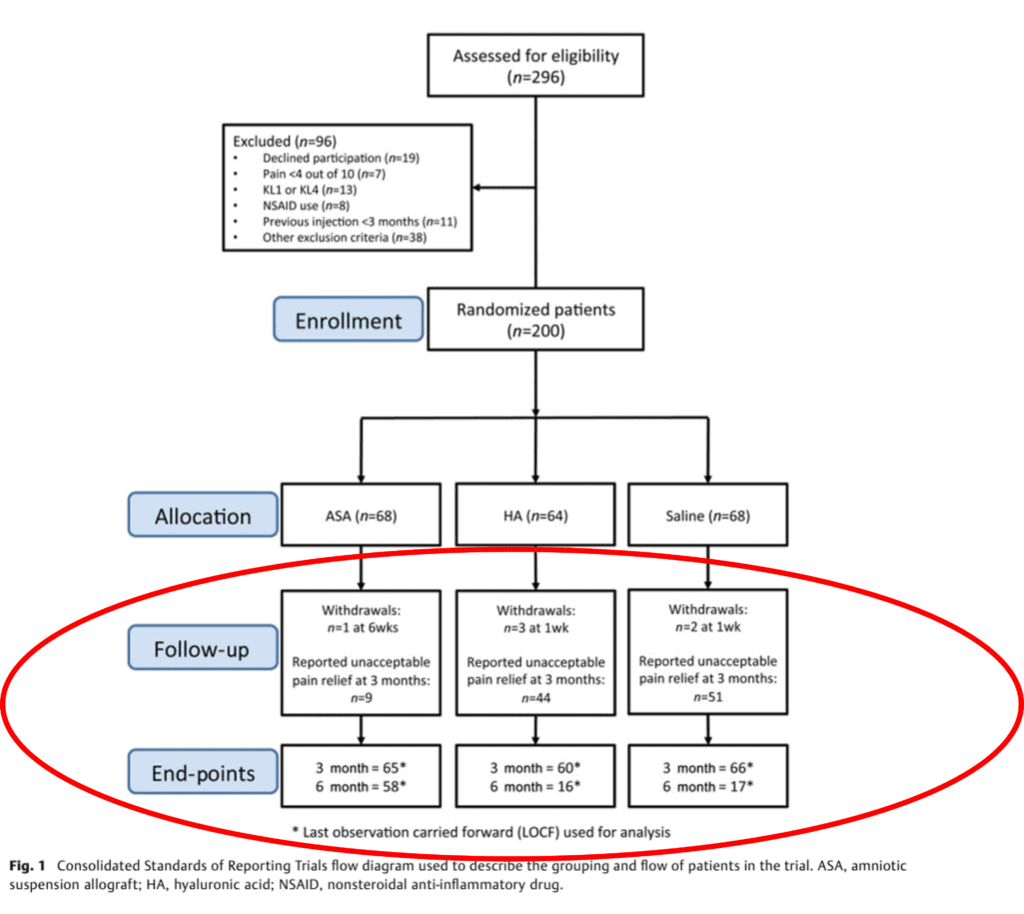
In the ReNu group, the company lost 15% by the end of the study, which is a bit high for an RCT, but still within the acceptable range. However, in the HA group, they lost 75% of the subjects! It was the same in the saline group where they also lost 75%! Those are in the very much “unacceptable” range for an RCT. Meaning you have a total of 109 subjects in a 200 patient trial where you have missing data. That’s more akin to a registry-based case series than an RCT.
The authors claimed this happened due to “unacceptable” pain relief at 3 months. I could buy that for saline, but HA is a known knee OA treatment that works reasonably well in moderate knee OA. Hence, the idea that 75% of the patients that received HA had unacceptable relief at 3 months isn’t clinically tenable. Meaning that doesn’t fit with what we see in the clinic all day long with patients who get HA shots.
ReNu Study #2-12-Month Data
Until I began searching for the 2019 study, I didn’t know that a 12-month study in the same group of patients existed. That study was published this summer (3). What did it show?
First, more dropout of subjects! Now we’re down to only 81 of the original 200 that began the study. That’s a loss of 24% in the ReNu group, 77% in the HA group, and 79% in the saline group!
Second, now the data looks MUCH better. There are now major differences in the questionnaires at 12-months that just weren’t there at 3 and 6 months. Which is curious. Why?
In orthobiologic studies, you can certainly see patients that take 3 or 6 months to show improvement. You can even see incremental improvements in patients after 6 months. What hasn’t been reported based on what I have reviewed is a massive number of patients who weren’t reporting improvement at 3 and 6-months who suddenly report dramatic improvement at 12-months.
So why did the authors likely see these effects? One explanation is fatal dropout. You kept far more of the ReNu group in the study and almost all of the control group subjects were gone. This is called selection bias.
The upshot? I know this was a long one, but I’m glad I did this keep dive into this amniotic knee osteoarthritis study. Prior to spending hours looking at these manuscripts, I would have counted myself a fan of ReNu. I still may be a fan, but not based on this published data. The company does have a phase III FDA pivotal trial that began in January of this year. So I’ll wait to see if Organogenesis publishes something else on the effects of amniotic tissue on knee arthritis.
______________________________
(1) Farr J, Gomoll AH, Yanke AB, Strauss EJ, Mowry KC; ASA Study Group. A Randomized Controlled Single-Blind Study Demonstrating Superiority of Amniotic Suspension Allograft Injection Over Hyaluronic Acid and Saline Control for Modification of Knee Osteoarthritis Symptoms. J Knee Surg. 2019 Nov;32(11):1143-1154. doi: 10.1055/s-0039-1696672. Epub 2019 Sep 18. Erratum in: J Knee Surg. 2019 Nov;32(11):e2. PMID: 31533151.
(2) Joshi Jubert N, Rodríguez L, Reverté-Vinaixa MM, Navarro A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop J Sports Med. 2017;5(2):2325967116689386. Published 2017 Feb 13. doi:10.1177/2325967116689386
(3) Gomoll AH, Farr J, Cole BJ, Flanigan DC, Lattermann C, Mandelbaum BR, Strickland SM, Zaslav KR, Kimmerling KA, Mowry KC. Safety and Efficacy of an Amniotic Suspension Allograft Injection Over 12 Months in a Single-Blinded, Randomized Controlled Trial for Symptomatic Osteoarthritis of the Knee. Arthroscopy. 2021 Jul;37(7):2246-2257. doi: 10.1016/j.arthro.2021.02.044. Epub 2021 Mar 12. PMID: 33716121.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.