Is American Cell Technology the Second Coming of US Stem Cell?
One of more interesting and not helpful chapters in regenerative medicine was when a company called US Stem Cell blinded three patients by injecting fat stem cells into their eyes. The company last year lost its battle with the FDA and let go of their fake Ph.D. Chief Science Officer, but recently a colleague asked me to look at a new company with a similar name. The more I dug, the more it looks like US Stem Cell could be reborn? Let’s dig in.
What Is/Was US Stem Cell?
You may have read about a company called US Stem Cell in the context of patients who were blinded by stem cells being injected into their eyeballs. Yes, you read that right, someone did actually inject stem cells from fat into the eyes of patients with macular degeneration leading to catastrophic results. As a result, US Stem Cell became the poster child for what was wrong and dangerous about an increasingly out of control stem cell clinic industry. So what’s the back story?
More than a decade ago, I came in contact with a cell biologist by the name of Kristin Comella, in an early iteration of what would become US Stem Cell. At the time, she worked as the lab manager for a company called Bioheart that was trying to get FDA approval to treat dead or weak heart muscle with cardiac muscle cells grown in the lab. The company was struggling financially and also had several FDA hiccups, all expensive. Hence Kristin decided to create her own company offering stem cells derived from fat for a number of maladies. Initially, she worked with a local family physician but later decided to work with Bioheart to rename the company, US Stem Cell. The focus shifted from FDA approval of heart muscle cells to treating many different incurable diseases by using mostly IV infusion cells derived from fat. This means the company harvested the patient’s own fat tissue through liposuction and then digested it with an enzyme to isolate the stem cell fraction in the fat (called Stromal Vascular Fraction or SVF). That quickly devolved into having a physician who had trained in Cuba, but who couldn’t be licensed in the US as a doctor, injecting fat stem cells into eyeballs. The rest as they say is history.
Getting Busted Doesn’t Mean You’re Dead?
The FDA eventually filed suit against US Stem Cell because it was using fat stem cells to treat many different types of serious incurable diseases. The agency considered this the manufacturing of a drug that would require FDA approval. The company fought the FDA and lost last year. However, what if US Stem Cell’s cell culture business still existed? What if despite an FDA order to shut down, the services of the company still existed? Could any company be bold enough to thumb it’s nose at the FDA twice?
What Is Cell Culture?
You can grow stem cells in a lab to get a larger number of cells. This is called cell culture or culture expansion. US Stem Cell used to offer this service as well. This is definitely not something the FDA permits, even within the confines of a single physician practice and even if the cells are from the same patient, as we found out the hard way many years ago. However, that didn’t stop US Stem Cell from breaking the law by offering this service to various physician offices. Eventually, the FDA caught up with US Stem Cell and decided to shut this operation down, which led to patients who had stored their stem cells to be told that the money they paid would go to waste and that their cells would be destroyed.
American Cell Technology (ACT)
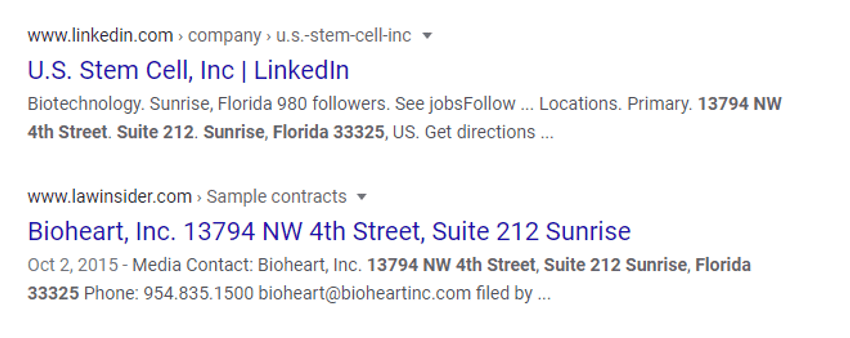
This company came on my radar after a colleague had a patient who was insistent that his doctor send his cells to ACT in Sunrise, Florida to be culture expanded. The doctor reached out because he knew that this wasn’t legal, but this was a new company name to him. The first thing I did was a reverse address look-up and this is what Google returned at the ACT address:
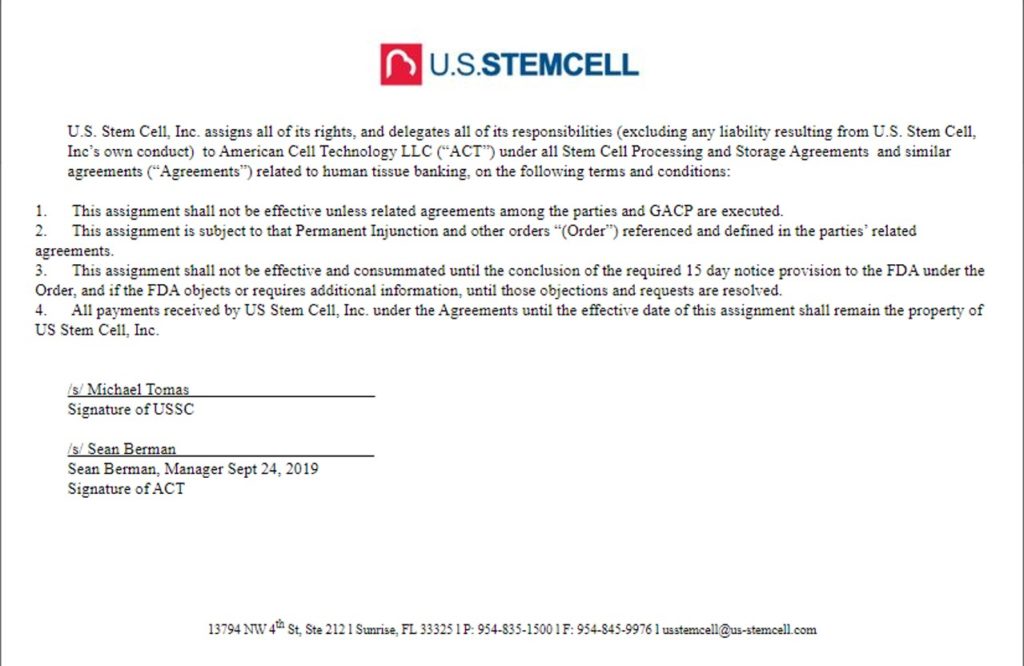
So American Cell Technology, US Stem Cell, and Bioheart all share the same address. Hmmm…. I then searched SEC documents for US Stem Cell and American Cell Technology and found this document filed with the government agency:
So US Stem Cell assigned its company assets to ACT. It somehow notified the FDA of the assignment, and the new company ACT was still subject to the permanent injunction that FDA had against US Stem Cell. Now here’s the confusing part, as a federal judge ordered US Stem Cell to destroy all cryopreserved patient samples, so clearly the judge didn’t agree that any of this was legal:
“Within thirty (30) calendar days after the entry of this Order, Defendants, under FDA’s supervision, shall destroy any and all SVF Product that is in Defendants’ possession, custody, or control. Defendants shall bear the costs of destruction and the costs of FDA’s supervision. Defendants shall not dispose of any SVF Product in a manner contrary to the provisions of the Act, any other federal law, or the laws of any state or territory, as defined in the Act, in which the drugs are disposed.”
What Is ACT?
ACT’s website says:
“American Cell Technology is a leading regenerative medicine company that provides stem cell banking services to collect, process, grow and store adult and newborn autologous Mesenchymal Stem Cells (MSCs) for future medical or therapeutic use.”
Huh? This is in part, what got US Stem Cell got busted for. Meaning you can’t culture expand stem cells in a lab and use them clinically without an FDA drug approval. Hence if ACT took over US Stem Cell, are they duplicating this business plan?
Enter Sean Berman
The agreement above was signed by Sean Berman. Who’s that? Well, Berman is the last name associated with another case that FDA brought against fat stem cell clinics. Mark Berman, M.D. is one of the principles of a company called Cell Surgical Network (CSN). While CSN was doing the same thing with fat stem cells as US Stem Cell (creating SVF and using it to treat a panoply of incurable diseases and offering to culture cells as well) and was also the subject of an FDA lawsuit, they won round 1 of their FDA court battle with the bigger court case pending. So is there any relation between Mark Berman and Sean Berman?
This is from Mark Berman’s personal website:
“Since starting our investigative network in 2012, we’ve not only gone to the animal lab for the Ommaya reservoir program, we’ve expanded our research into areas of cancer, paralysis, and most recently, concussion. My son, Sean, in fact, has been doing some terrific animal research in the area of concussion where he’s been able to first, induce reproducible concussions in trained animal…”
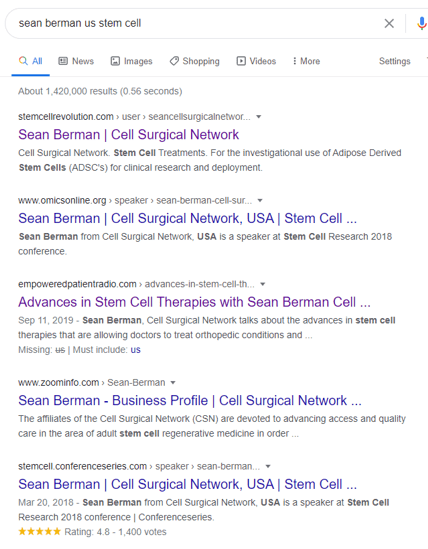
I also did a Google search for Sean Berman and CSN and got these hits:
Hence, it’s highly likely that Sean Berman, the son of CSN owner Mark Berman bought the cell expansion and banking business of US Stem Cell and turned it into ACT.
The New Scientific Director
Given that the last scientific director of US Stem Cell (Kristin Comella) had a fake PhD. from a Panamanian college that didn’t really exist, how does the new one for ACT measure up? We’re met with this imposing picture:
The website goes on to say: “Dr. Elena Rusyn is medical scientist dedicated to enhancing the quality and extending the length of the human lifespan. She is Board Certified in Anti-Aging and Regenerative Medicine and has more than 8 years of experience in stem cell research and over 20 years of medical and clinical research training.” So let’s dig in.
After finding that Dr. Rusyn was trained in Ukraine, I wanted to make sure she was licensed as a physician in the US. Hence, I first performed a State of Florida Medical License look-up. There is no Florida licensed physician with that name. I did find a Google hit in California for Dr., Rusyn. Hence I then searched the California state medical board site and also found no medical license.
Dr. Rusyn claims to have a “Board Certification”. I then went to the American Board of Medical Specialities (ABMS) and found no board certifications for a physician with that name. I then noticed that the “Board Certification” in regenerative medicine she claims to have is in fact not a board certification, but instead a certificate from a course offered by a group called A4M.
This all made no sense, but on Dr. Rusyn’s website I found this:
“Disclaimer: Dr. Rusyn is currently actively applying for medical residency in the State of California in order to complete her medical license requirements. She is not practicing physician and does not offer any services that can be considered as “practice of medicine”. Your treating physician is one of our licensed and board-certified network providers.”
So finding no medical license or board certification was spot on.
Maybe Dr. Rusyn is a stem cell scientist who has lots of expertise in this area of regenerative medicine, despite not having a medical license? After all, she claims that she has 8 years of experience in stem cell research. Let’s vet that claim.
I first ran a search under “Rusyn E” and “stem cells” in the US National Library of Medicine. I found no publications. I searched under that name and platelets and also found no publications. I ran similar searches for bone marrow, adipose, and stromal vascular fraction with the same results. I did eventually find a CV on the Cell Surgical Network site (further connecting ACT to CSN?) which lists papers in cell biology, cancer, and pediatric diseases but not in the field of regenerative medicine. Of note, she claims about 20 papers (the newest is 2010) where she is on the author list, but in only two is she the lead author. Those two papers are about pediatric disease and cancer. Is there anything in the past 8 years on stem cells as the ACT website claims? Nope. Dr. Rusyn appears to have experience in cell biology, pediatric diseases, and cancer research but is not a published expert in the science of regenerative medicine.
In conclusion, the person that ACT offers as a chief scientist and as a “Board-certified” M.D., Ph.D.” is actually an unlicensed Ukrainian physician with no board certifications and no publications that would demonstrate subject matter expertise in stem cells or regenerative medicine.
What is ACT Doing with US Stem Cell’s Assets?
I can’t confirm that ACT is permitting anyone to use these cells that they culture and store. If they are, then based on my understanding, this clearly breaks the law just like US Stem Cell. If not, then they’re offering a service that can never be used. Meaning, to be able to use these cells in patients, the company would have to have an FDA drug approval for every clinical indication. Not even Pfizer could afford the billions it would take to jump that bar and get FDA approval for 20-30 medical indications. The company also can’t legally export an unapproved drug product (ship the cells to another country). Hence, in my opinion, if ACT is playing within the boundaries of the law, it’s offering a product that’s like an insurance policy that can never pay off.
The upshot? The fact that US Stem Cell sold their stem cell culture and banking business to the Cell Surgical Network owner’s son is as crazy as it gets. The fact that they’re still openly offering to culture cells is crazier. The fact that their new Chief Scientific Officier is a Ukrainian physician with no medical license or stem cell publications is also nutty. You just can’t make this stuff up!
__________________________________________
6/26/20-I have been contacted by the attorney for ACT. He asserts that ACT is not connected with Cell Surgical Network.
8/28/20-A prior version of this blog erroneously stated that ACT shared counsel with US Stem Cell, Inc. Counsel for ACT is in fact also counsel for Cell Surgical Network.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.