Very Early Shoulder Rotator Cuff Stem Cell Study Data
As many of my readers know, we have sponsored several different randomized controlled trials using stem cell injections to treat several different orthopedic conditions. One of those is a rotator cuff stem cell study. This study is about 1/3 through it’s recruitment goal and we have some 6 month data, so I’ll report it here as very preliminary.
First, the study focuses on partial through full thickness non-retracted rotator cuff tears. This is a randomized controlled trial where there is no charge to the patient and 3-D volume acquisition ultrasound is used to access the tear (which won’t be reported until the study is complete). The patients are randomized to either a control group where they must wait 3 months while receiving physical therapy or they begin the active treatment, with the control group crossing over to the active group if they still have issues at 3 months. The active treatment is the Regenexx-SD protocol for rotator cuff where the cells are placed under precise ultrasound guidance into the tears.
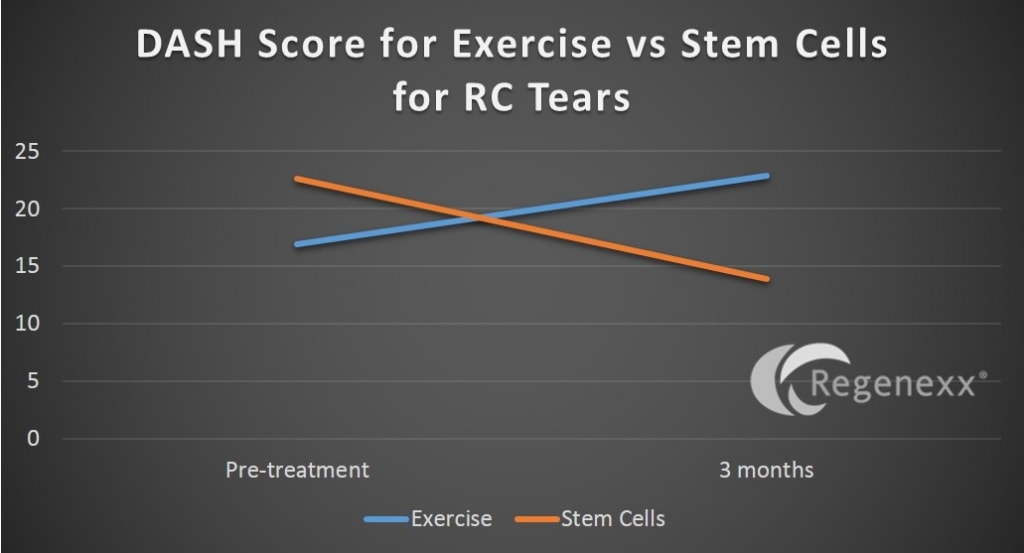
First, this data is very preliminary, with only a handful of patients reaching the 6 month mark where we can get a bead on what they reported on a standardized questionnaire (DASH) in either the active or control group. Having said that, large publicly traded stem cell companies hype small randomized data sets all the time to pump the stock price, so I thought we might as well do the same (without any stock price to pump).
From the graph above, 6 patients have reached the critical 6 month mark. That means 3 patients were randomized to do their exercises and then were treated with stem cells at 3 months and 3 were randomized to get the therapy and then have been tracked for 6 months. The fact that the therapy group above shows more disability (the exercise line goes up) and the stem cell treated group shows less disability (the stem cell line goes down) fits with what we see clinically.
The upshot? Looking at very early data (this could change once we look at more patients), the shoulder rotator cuff stem cell injection using our very unique protocol seems to be helping patients. More data is needed! In addition, since patients know they’re being treated and when, the final arbiter of this type of study may have to be the changes in objective imaging (hence the 3D volume ultrasound we have been performing on these patients). In the end, that data will be sent to a number of blinded readers who don’t know which image is from before and which is from after the treatment or even if the patient was treated at all. In the meantime, if you know of anyone who would be interested in getting an expensive stem cell procedure for no charge who has a rotator cuff tear and is willing to be randomized, have them contact [email protected]!

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.