Tony Robbins Life Force Review
I’ve been getting lots of emails lately about the Tony Robbins book “Life Force”. It’s always interesting as a physician to get bombarded by patients with what sounds like misinformation, but since you haven’t looked at the source, you’re not sure how to respond. In fact, patients come in with new things every day, so medical practice these days is a constant task of looking it all up on the Internet. Hence, I’d like to take a deeper dive into a recent interview with Tony Robbins and his book to see for myself what he’s saying about stem cells. Let’s dig in.
What is Life Force?
I downloaded Tony’s new book “Life Force” onto my Kindle. It’s 600+ Kindle pages and I’m a busy doctor, so I used it as a cross-reference for an interview a colleague sent me. I also looked through the book to get a sense of its content.

In Tony’s own words, in the book, he interviewed approximately 150 experts in regenerative medicine and new medical trends. This runs the gamut from stem cell therapy to new ultrasound treatments for Parkinson’s disease. Apparently, the story behind the title is Tony’s fascination with placental and umbilical tissue which he feels has a vigorous “life force”.
On the one hand, the good news is that Tony’s book is educating people about the whole space of regenerative medicine. On the other, I keep getting patients coming in quoting Tony and that information sounds wildly inaccurate. So let’s see what’s actually being said.
A Tony Robbins News Interview
Let’s face it, while Tony may sell millions of copies of this book, most people will likely only ever see his interviews about the book on social media. For the most part, it’s what Tony says during these interviews that turn into the patient questions I get in the office. Hence, I was recently sent a 9-minute interview that Tony Robbins did with Fox 5 New York which I watched and forms the core of this review. I’ll only focus here on five statements that would be of interest to my patients who have orthopedic problems.
This is what Tony says during the interview:
- Tony had spinal stenosis and a completely torn rotator cuff and an IV of umbilical derived stem cells and “a shot” cured him in a few days (this statement is a composite of what he said in the interview).
- If you use your own stem cells and you’re over the age of 35 or 40 they “drop off a cliff”.
- Literally, you can heal in days instead of weeks or months.
- If you have arthritis, there is a company in a phase III trial that will be approved by FDA by this year or next and a single shot regrows all of your tendons in 11 months. It doesn’t matter if you’re 40 or 80, you get 16-year-old tendons.
- Reporter-Are there risks to this? Tony-The FDA has approved stem cells for over 80 different diseases.
Let’s take these statements one by one.
Before we do that, let’s differentiate what Tony is talking about here from what is commonly offered in US clinics. Tony is discussing the isolated and culture-expanded umbilical cord derived stem cells he received OUTSIDE of the U.S. That’s very different than the umbilical cord products offered to patients HERE in the United States, which our published research demonstrated contain NO living and functional stem cells (36).
Tony had spinal stenosis and a completely torn rotator cuff and an IV of umbilical derived stem cells cured him in a few days.
Tony claims that he had been diagnosed with a complete rotator cuff tear requiring surgery and had chronic low back pain from severe spinal stenosis and after being hooked up to an IV of umbilical cord-derived stem cells he was healed in a few days.
Could IV stem cells have healed his shoulder and low back in a few days?
First, is injecting stem cells via a vein a good idea? There is a pulmonary first-pass effect when giving IV mesenchymal stem cells (MSCs). That means that 95% get stuck in the lungs (1). Of the 5% left, rotator cuff tears are generally avascular or have poor blood flow, so the stem cells getting to the right spot in the rotator cuff is unlikely via the arteries/veins. That’s doubly true if there are gaps in the tissue, which would be common with complete rotator cuff tears. So any remaining stem cells likely wouldn’t get where you need them to be, which would be in the torn parts of the rotator cuff.
How about “the shot”? Tony said on Kindle page 52 of his book that he had three injections into his rotator cuff. On day three his shoulder and low back were pain-free. So while this could have delivered cells locally to the rotator cuff tear, depending on how this procedure was performed, none of those cells are capable of regrowing tendons in three days (more on that below). If Tony got any effect in that time period it was likely anti-inflammatory or a placebo effect.
Next up we have spinal stenosis. Tony says that he had chronic low back pain and that a spinal specialist told him that his life of being active was over. This was apparently due to severe spinal stenosis, as the doctor told Tony that he might not be able to walk with “one good hit”. Spinal stenosis is when the spinal canal or areas where the nerves exit (foramina) are too tight due to bone spurs, overgrown ligaments, or disc bulges. This irritates the nerves and causes pain.
Tony doesn’t mention that any injections were performed in the spine, just on his shoulder. So how did the cells make it to this area? They really wouldn’t have made it there. First, the constricted nerves also have a poor blood supply as that’s what’s getting constricted. So any stem cells hoping a ride in arteries or veins likely wouldn’t make it to these nerves. This disc is also largely avascular, so it only has a few small vessels where stem cells could hitch a ride. The bone spurs could be reached, but changing their shape with stem cells has never been documented and that takes at least months.
As an expert with dozens of peer-reviewed publications in this field, do either of these recovery stories sound credible? On the one hand, we do see some orthobiologics patients recover quickly because of the anti-inflammatory effects of orthobiologic injections. But healing tendons doesn’t happen in days. Nor will IV stem cells make it into the stenotic areas of the spine that cause pain, nor will they cure stenosis. Could there have been some form of whole-body anti-inflammation? Possible. However, after following many patients who have received IV umbilical stem cell injections over the years, some report responding and many don’t.
When you search the US Library of Medicine to see if there is ANY published research on using IV umbilical cord stem cells to treat spinal stenosis, you get this result:
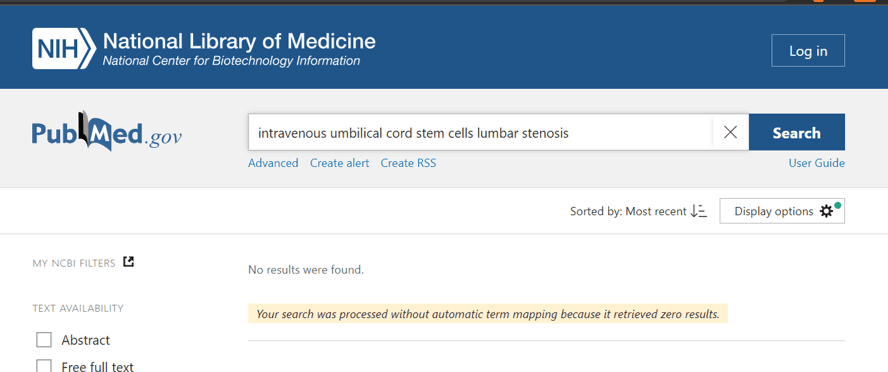
There is no research at all on this topic, not even in animal models.
While we have treated spinal stenosis using regenerative medicine techniques, that requires sophisticated x-ray and ultrasound-guided direct injection of the stenotic area. These are technically demanding procedures to perform, so you would know if you had them done. For example, using ultra-precise x-ray guidance to get the orthobiologic into the hypertrophied ligamentum flavum.
If you use your own stem cells and you’re over the age of 35 or 40 they “drop off a cliff”.
Is there any truth to the statement that once you’re over the age of 35-40 your stem cells are too old to be used? In short, NO. We have no credible data from clinical studies on orthopedic problems using various types of stem cell-based orthobiologics that show that your stem cells can’t be used once you’re over that age. For example, we have published several studies using the patient’s own bone marrow concentrate (autologous) for knee osteoarthritis and we have never seen an age-related impact on outcomes (2-4). In fact, we have the world’s largest registry for orthopedic care going back to 2005 and we have never seen that relationship in about 8,000 treated knee arthritis patients. In addition, Phillipe Hernigou just published a 15-year follow-up on middle-aged and elderly knees showing that 80% of the patients avoided a knee replacement by using injections of their own bone marrow concentrate (which contains stem cells) (5). In fact, in another study, the autologous bone marrow treated knees did better than the comparison knee that was replaced (6).
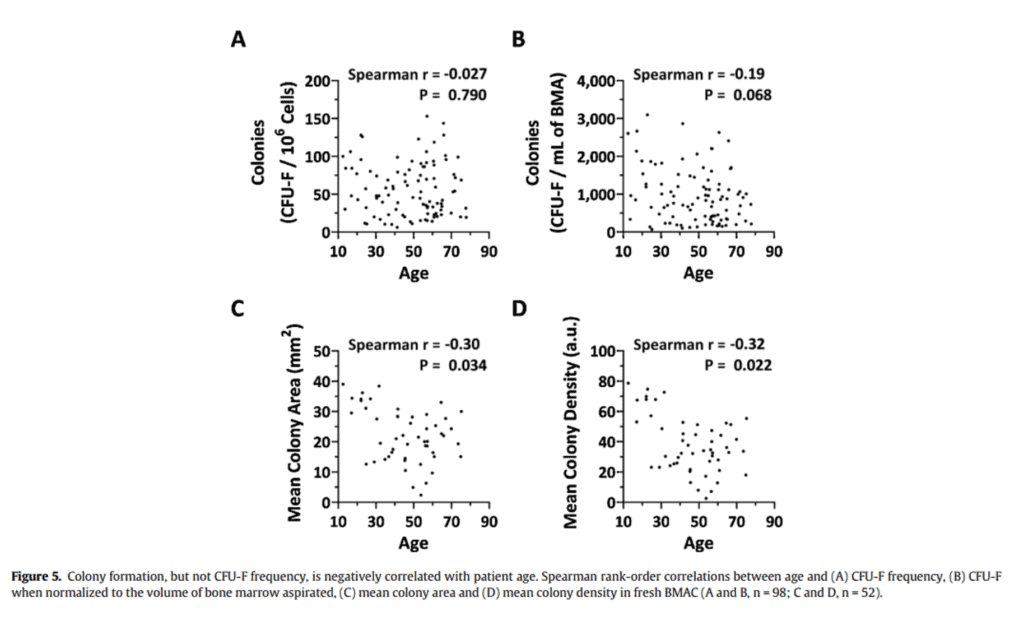
Is it true that your stem cell count in your bone marrow “falls off a cliff” as you get older than 35-40? Not based on one of the world’s largest research studies that was published by our research team (7). In figure 5 from our paper above, the number of stem cells in young versus older bone marrow wasn’t correlated to age (colony formation). In fact, if you look at studies that show an age-related decline in absolute stem cell numbers, it’s often because the authors included babies and the very young and didn’t compare a 30-year-old to a 40-year-old.
What does change with age is the speed of cell growth, but that data doesn’t support Tony’s statement. More on that below.
Literally, you can heal in days instead of weeks or months.
In my opinion, this is where things really come off the rails. There are time barriers in healing tissue that have not been broken in any study using stem cells that I have ever found. Meaning healing something like a tendon must take a minimum amount of time based on how fast the fastest cells can work. So while some magic not yet invented may speed this up in the future, this statement IMHO is more science fiction at this point than science fact. Let’s explore why.
Cells grow at certain rates. While very young cells can grow faster than old cells, all cells grow within a range called their “Population Doubling Time” (PDT). That’s how long it takes the cell number to double when the cells are in culture. For example, a number of studies on umbilical cord MSCs derived from Wharton’s Jelly showed a PDT of 23-39 hours (8). While that’s shorter than the PDT of most bone marrow mesenchymal stem cells from adults, it’s not magic. For example, we have patients who grow their MSCs down in our licensed Cayman lab who have PDTs in the 2-3 day range. Either of these cell types, when assisting the healing of something like a tendon, can’t heal that structure in days. There is nothing about the math of how fast cells can grow and lay down new tissue that adds up to that result.
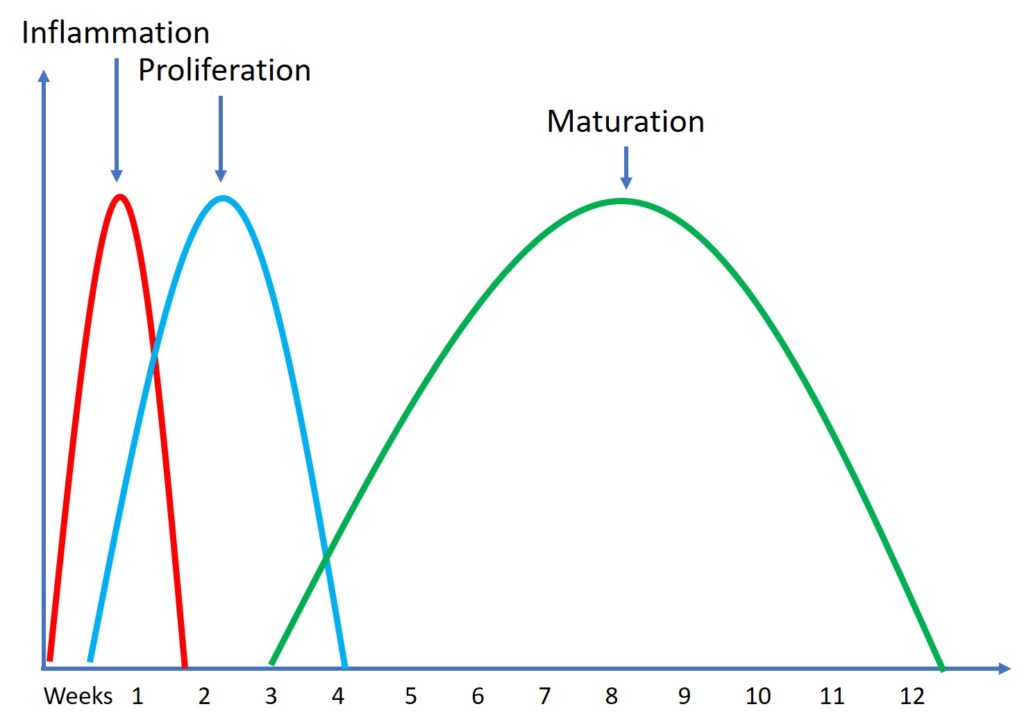
For example, we know we have three very predictable tendon healing cycles that happen in everyone, even in babies or the very young (30). First, there is inflammation, which lasts a few days. This is where new cells are brought into the area. Then there is proliferation where the cells help new tissue to be laid down and this lasts a few weeks. Then finally we have maturation and remodeling of that tissue which takes a few months. While the young can do all of this faster than the middle-aged, after treating many young people while they heal, their tendons don’t recover fully in days.
Now let’s look at the clinical data published to date on using umbilical cord MSCs to see how quickly they can heal a damaged rotator cuff tendon. For example, a US National Library of Medicine search under the terms “Umbilical Cord Stem Cells Rotator Cuff” yields only 11 studies, ALL performed on rats or rabbits (meaning there is no published human research that I found). Let that last statement sink in a bit. I couldn’t find a single human study on using umbilical cord drevied MSCs to treat torn rotator cuffs. On the other hand, there are lots of positive human clinical studies out there on using PRP or autologous bone marrow concentrate to help rotator cuff healing (33-35).
Even in rats where tendons heal much faster than in humans, the studies still show a month or more of healing time (9). In addition, it turns out that in a rabbit, an animal that also has quicker healing times than middle-aged humans, the healing time is also about 4 weeks (10). That’s likely not complete healing and remodeling of the tendon, just a time point where researchers can see changes in animals that have lifespans measured in months.
In conclusion, we have no basic science or clinical data that shows that it’s possible to heal a damaged tendon like the rotator cuff in days rather than weeks or months.
If you have arthritis, there is a company in a phase III trial that will be approved by FDA by this year or next and a single shot regrows all of your tendons in 11 months. It doesn’t matter if you’re 40 or 80, you get 16-year-old tendons.
The company Tony seems to be discussing here is Biosplice, a gene therapy company working on a drug candidate with the FDA. Tony, in his book (Kindle pages 195-198) tells us how Biosplice’s drug candidate can regrow new cartilage in knees, “Imagine what it would feel like to regenerate your worn cartilage and walk or run without so much as a twinge.” What I got out of what I read there is that Biosplice’s product can regrow large amounts of new cartilage in aging knees that have lost that cartilage.
Now let’s look at what really happened in Biosplice’s phase II clinical trial (they are now in phase III) (11). First, the study didn’t measure any changes in x-rays or MRIs with regard to regrowing cartilage. In addition, the short-term benefits of Biosplice’s product were very modest:
“With this composite responder index, about 70% of 0.07 mg LOR participants achieved a moderate response at weeks 12 and 24. Approximately 56% of the LOR-treated group achieved a large response at weeks 12 and 24. These results were each statistically significant compared with PBO at both timepoints.”
So about 7 in 10 got moderate results at 6 months out and a bit more than half had a larger response. However, when you look at Biospice’s product compared to the placebo, the results are even less impressive:
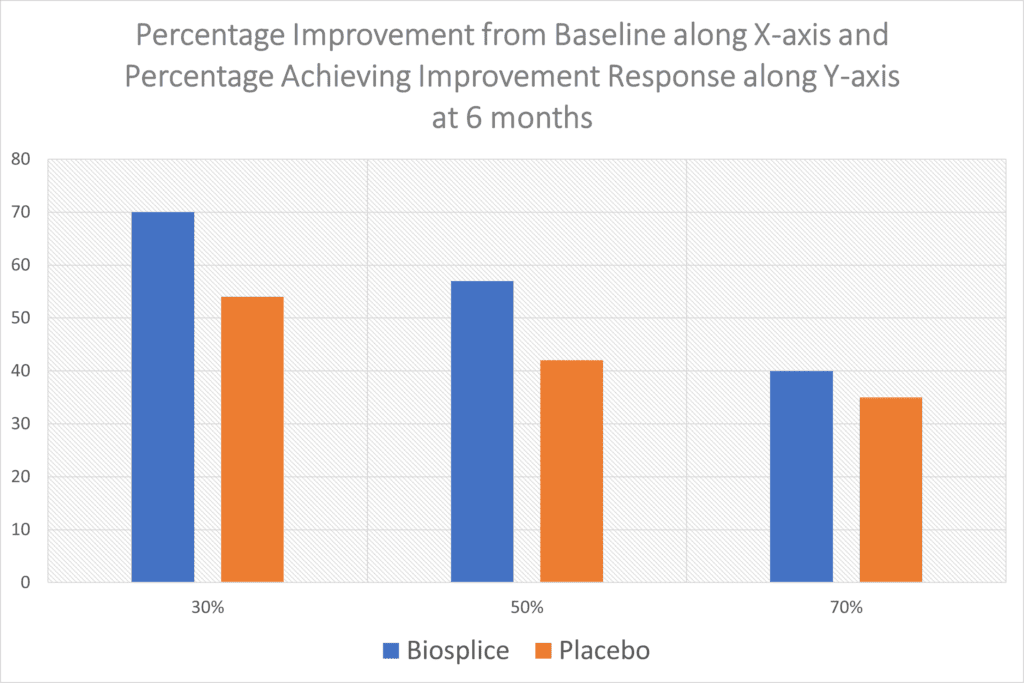
I took the Biosplice WOMAC pain data and graphed it above, as the table from the paper online is pretty grainy. There’s just no way around it, the results above are poor. 54% of the patients who got a placebo knee injection reported a meager 30% improvement in pain (WOMAC Score) while 70% who got the drug reported this level of improvement. At the 50% improvement level, you can see the drug and placebo are even closer and then the two are almost indistinguishable at the 70% improvement level. None of this improvement at 6 months is consistent with regrowing large amounts of new cartilage.
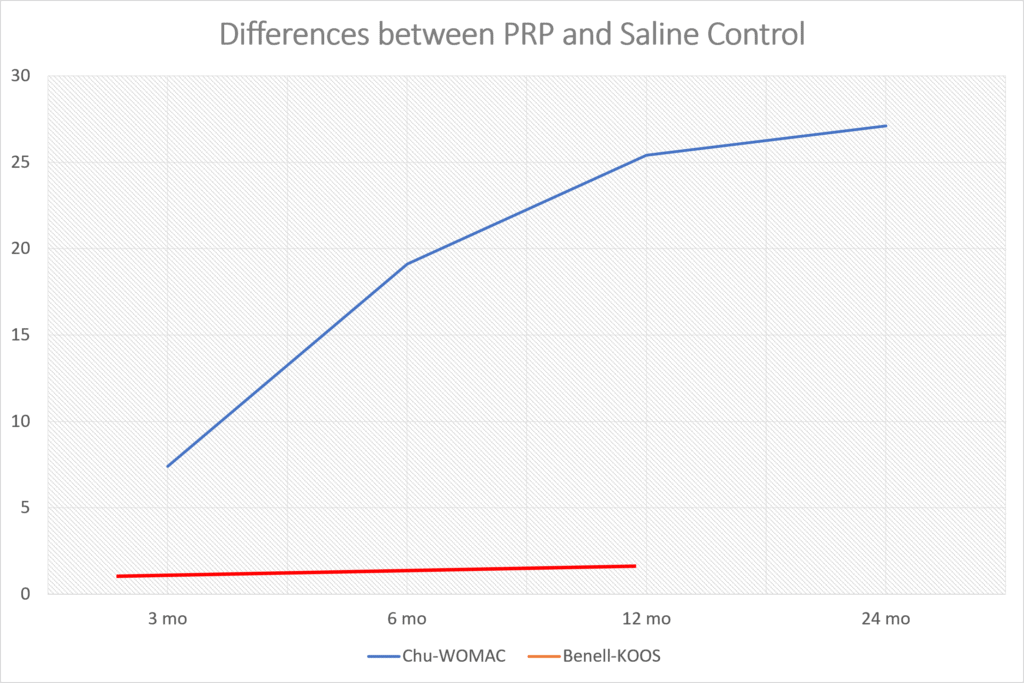
Frankly, from looking at the data they published, there was a smaller response to their product than the reported results in many randomized controlled trials of MUCH CHEAPER platelet-rich plasma (PRP) (13-29). For example, let’s take a look at the results from one recent PRP knee arthritis paper. Above I graphed the differences between the saline control (Chu for the WOMAC) and PRP (blue line) (31). Those differences get bigger between 3 and 6 months and not smaller as they do in Biosplice’s trial. The red line here is a recent study that used a very low dose platelet concentration that didn’t meet the minimum definition of PRP and saw no difference between the treatment and placebo (32).
Finally, if you read this blog, you also know that knee arthritis is a disease of bone and cartilage. Hence Tony’s statement about regrowing tendons to a 16-year-old level is nonsensical in this context. In addition, if he meant to say cartilage, the above phase II FDA clinical trial data for Biosplice doesn’t support that cartilage was regrown to a 16-year-old level as the clinical effects of the drug barely beat the placebo.
Reporter-Are there risks to this? Tony-The FDA has approved stem cells for over 80 different diseases.
This was an easy one to fact check. This is posted on the FDA website (12):
“About FDA-approved Products Derived from Stem Cells
The only stem cell-based products that are FDA-approved for use in the United States consist of blood-forming stem cells (hematopoietic progenitor cells) derived from cord blood.
These products are approved for limited use in patients with disorders that affect the body system that is involved in the production of blood (called the “hematopoietic” system). These FDA-approved stem cell products are listed on the FDA website.”
Basically, per the FDA, stem cells are only approved to treat pediatric cancer. So there is no evidence that the FDA has approved stem cell therapies to treat over 80 different diseases.
Summing it All Up
To recap, I have been getting bombarded by patients and colleagues who mostly have heard Tony Robbins being interviewed on social media about the new book. Hence, it was high time that I took a look for myself. As you can see, in my opinion, the five statements that Tony made in this interview can’t be supported by the science behind regenerative medicine or what the FDA reports. From looking through the book, some of his statements are a bit more grounded and there are some super interesting technologies discussed. However, in my opinion, some of the claims in the book are as wild as the claims he makes in this interview.
The upshot? I’m sure Tony is a great personal transformation speaker and obviously, he has a passion for whatever he does. However, at this point, my concern is that my patients are seeing his interviews on social media, and in my opinion, they are getting wildly misinformed.
______________________________________________________________
References:
(1) Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009 Jun;18(5):683-92. doi: 10.1089/scd.2008.0253. PMID: 19099374; PMCID: PMC3190292.
(2) Centeno C, Sheinkop M, Dodson E, Stemper I, Williams C, Hyzy M, Ichim T, Freeman M. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med. 2018 Dec 13;16(1):355. doi: 10.1186/s12967-018-1736-8. PMID: 30545387; PMCID: PMC6293635.
(3) Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015 Sep 18;16:258. doi: 10.1186/s12891-015-0714-z. PMID: 26385099; PMCID: PMC4575428.
(4) Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. doi: 10.1155/2014/370621. Epub 2014 Sep 7. PMID: 25276781; PMCID: PMC4170694.
(5) Hernigou P, Delambre J, Quiennec S, Poignard A. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: a prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int Orthop. 2020 Apr 23. doi: 10.1007/s00264-020-04571-4. Epub ahead of print. PMID: 32322943.
(6) Hernigou P, Bouthors C, Bastard C, Flouzat Lachaniette CH, Rouard H, Dubory A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? A randomized study. Int Orthop. 2020 Jul 2. doi: 10.1007/s00264-020-04687-7. Epub ahead of print. PMID: 32617651.
(7) Berger DR, Aune ET, Centeno CJ, Steinmetz NJ. Cryopreserved bone marrow aspirate concentrate as a cell source for the colony-forming unit fibroblast assay. Cytotherapy. 2020 Sep;22(9):486-493. doi: 10.1016/j.jcyt.2020.04.091. Epub 2020 Jun 19. PMID: 32565131.
(8) Hendijani F, Sadeghi-Aliabadi H, Haghjooy Javanmard S. Comparison of human mesenchymal stem cells isolated by explant culture method from entire umbilical cord and Wharton’s jelly matrix. Cell Tissue Bank. 2014 Dec;15(4):555-65. doi: 10.1007/s10561-014-9425-1. Epub 2014 Feb 17. PMID: 24532125.
(9) Yea JH, Kim I, Sym G, et al. Regeneration of a full-thickness defect in rotator cuff tendon with umbilical cord-derived mesenchymal stem cells in a rat model. PLoS One. 2020;15(11):e0235239. Published 2020 Nov 9. doi:10.1371/journal.pone.0235239
(10) Kwon DR, Park GY, Lee SC. Regenerative effects of mesenchymal stem cells by dosage in a chronic rotator cuff tendon tear in a rabbit model. Regen Med. 2019 Nov;14(11):1001-1012. doi: 10.2217/rme-2018-0125. Epub 2019 Nov 15. PMID: 31726959.
(11) Tambiah JRS, Kennedy S, Swearingen CJ, Simsek I, Yazici Y, Farr J, Conaghan PG. Individual Participant Symptom Responses to Intra-Articular Lorecivivint in Knee Osteoarthritis: Post Hoc Analysis of a Phase 2B Trial. Rheumatol Ther. 2021 Jun;8(2):973-985. doi: 10.1007/s40744-021-00316-w. Epub 2021 Jun 8. PMID: 34101138; PMCID: PMC8217418.
(12) USFDA. FDA Warns About Stem Cell Therapies. https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies. Accessed 4/26/22
(13) Uslu Güvendi E, Aşkin A, Güvendi G, Koçyiğit H. Comparison of Efficiency Between Corticosteroid and Platelet Rich Plasma Injection Therapies in Patients With Knee Osteoarthritis. Arch Rheumatol. 2017;33(3):273–281. Published 2017 Nov 2. doi: 10.5606/ArchRheumatol.2018.6608
(14) Tavassoli M, Janmohammadi N, Hosseini A, Khafri S, Esmaeilnejad-Ganji SM. Single- and double-dose of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: A randomized controlled trial. World J Orthop. 2019;10(9):310–326. Published 2019 Sep 18. doi: 10.5312/wjo.v10.i9.310
(15) Joshi Jubert N, Rodríguez L, Reverté-Vinaixa MM, Navarro A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop J Sports Med. 2017;5(2):2325967116689386. Published 2017 Feb 13. doi: 10.1177/2325967116689386
(16) Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) Versus Hyaluronic Acid (A one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. Published 2015 Jan 7. doi: 10.4137/CMAMD.S17894
(17) Montañez-Heredia E, Irízar S, Huertas PJ, et al. Intra-Articular Injections of Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System. Int J Mol Sci. 2016;17(7):1064. Published 2016 Jul 2. doi: 10.3390/ijms17071064
(18) Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017 Mar;25(3):958-965. doi: 10.1007/s00167-015-3705-6.
(19) Lana JF, Weglein A, Sampson SE, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69–78. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5227106/
(20) Tavassoli M, Janmohammadi N, Hosseini A, Khafri S, Esmaeilnejad-Ganji SM. Single- and double-dose of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: A randomized controlled trial. World J Orthop. 2019;10(9):310–326. Published 2019 Sep 18. doi: 10.5312/wjo.v10.i9.310
(21) Lin KY, Yang CC, Hsu CJ, Yeh ML, Renn JH. Intra-articular Injection of Platelet-Rich Plasma Is Superior to Hyaluronic Acid or Saline Solution in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized, Double-Blind, Triple-Parallel, Placebo-Controlled Clinical Trial. Arthroscopy. 2019 Jan;35(1):106-117. doi: 10.1016/j.arthro.2018.06.035.
(22) Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis : A prospective randomized controlled study. Orthopade. 2019 Mar;48(3):239-247. doi: 10.1007/s00132-018-03659-5.
(23) Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, Kon E, Filardo G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am J Sports Med. 2019 Feb;47(2):347-354. doi: 10.1177/0363546518814532.
(24) Yu W, Xu P, Huang G, Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med. 2018;16(3):2119–2125. doi: 10.3892/etm.2018.6412
(25) Buendía-López D, Medina-Quirós M, Fernández-Villacañas Marín MÁ. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol. 2018;19(1):3. Published 2018 Aug 20. doi: 10.1186/s10195-018-0501-3
(26) Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018 May;37(5):1341-1350. doi: 10.1007/s10067-018-3985-6.
(27) Louis ML, Magalon J, Jouve E, Bornet CE, Mattei JC, Chagnaud C, Rochwerger A, Veran J3, Sabatier F. Growth Factors Levels Determine Efficacy of Platelets Rich Plasma Injection in Knee Osteoarthritis: A Randomized Double Blind Noninferiority Trial Compared With Viscosupplementation. Arthroscopy. 2018 May;34(5):1530-1540.e2. doi: 10.1016/j.arthro.2017.11.035.
(28) Lisi C, Perotti C, Scudeller L, Sammarchi L, Dametti F, Musella V, Di Natali G. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil. 2018 Mar;32(3):330-339. doi: 10.1177/0269215517724193
(29) Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am J Sports Med. 2017 Feb;45(2):339-346. doi: 10.1177/0363546516665809.
(30) Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99(3):203-222. doi:10.1002/bdrc.21041
(31) Chu J, Duan W, Yu Z, Tao T, Xu J, Ma Q, Zhao L, Guo JJ. Intra-articular injections of platelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2022 Feb 6. doi: 10.1007/s00167-022-06887-7. Epub ahead of print. PMID: 35124707.
(32) Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A, Harris A, Yu SP, Connell D, Linklater J, Wang BH, Oo WM, Hunter DJ. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA. 2021 Nov 23;326(20):2021-2030. doi: 10.1001/jama.2021.19415. PMID: 34812863; PMCID: PMC8611484.
(33) Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, Rouard H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014 Sep;38(9):1811-8. doi: 10.1007/s00264-014-2391-1. Epub 2014 Jun 7. PMID: 24913770.
(34) Zhang C, Cai YZ, Wang Y. Injection of Leukocyte-Poor Platelet-Rich Plasma for Moderate-to-Large Rotator Cuff Tears Does Not Improve Clinical Outcomes but Reduces Retear Rates and Fatty Infiltration: A Prospective, Single-Blinded Randomized Study. Arthroscopy. 2022 Mar 3:S0749-8063(22)00093-7. doi: 10.1016/j.arthro.2022.02.007. Epub ahead of print. PMID: 35247512.
(35) Thepsoparn M, Thanphraisan P, Tanpowpong T, Itthipanichpong T. Comparison of a Platelet-Rich Plasma Injection and a Conventional Steroid Injection for Pain Relief and Functional Improvement of Partial Supraspinatus Tears. Orthop J Sports Med. 2021 Sep 1;9(9):23259671211024937. doi: 10.1177/23259671211024937. PMID: 34485587; PMCID: PMC8414632.
(36) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413. doi: 10.1177/03635465211031275. Epub 2021 Aug 16. PMID: 34398643.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.