Why Is Regen Med Out of Control? The A4M Conference Example

With all of the information in the media and that being distributed by the FDA going after “bad actors” in the stem cell wild west, why is the regenerative medicine space getting more and more out of control and not less? In my opinion, look no further than pay for play conferences. There are many of these, but one that I recently experienced first hand was A4M. Let me explain.
The Stem Cell and Exosome Wild West
These past 24-36 months have seen an explosion in regenerative medicine offerings. Everything from birth tissues that contain no live and functional stem cells, but are sold by clinics as “stem cell” therapies, to exosome products that are all hype with no clinical reality. Month over month, based on what I see online, this stem cell and exosome wild west is exploding.
On the other side of that equation, is the pressure from local, state, and federal regulators as well as the media which heads the opposite direction. For example, we’ve seen state medical boards begin to crack down. We’ve also seen countless FDA Warning and other letters to stem cell and exosome “bad actors”. We’ve seen FTC actions against amniotic “stem cell” clinics. The media is also constantly weighing in, often unable to tell good from bad actors, but just throwing everyone under the bus.
So how is it possible, with all of this pressure, that the stem cell and exosome wild west continues to grow like a weed? While there are many reasons, in my opinion, one the FDA should be seriously looking at is pay for play conferences. Let me explain.
What is a Pay for Play Conference?
Most patients and many physicians look at medical conferences as the highest level of medical education. They would believe that the physicians that speak at the podium in medical conferences are experts in their fields. This is mostly true when you’re dealing with nonprofit professional organizations. However, about 1-2 decades ago we began to see the rise of what I call “Pay for Play” conferences.
These conferences are different. Generally, if you purchase booth space or are a sponsor, you can get a podium presentation. In my direct experience, the more you spend, the bigger the exposure you can get. While some professional societies have also played this game, at pay for play conferences it gets extreme. Oftentimes you’re wondering why someone who is clearly not an expert is lecturing in front of hundreds to thousands of physicians.
My A4M Conference Experiences
My first experience with the American Academy of Antiaging Medicine was more than a decade ago. I was asked to speak on the orthopedic uses of stem cells but stuck around to watch the lectures which were on age management medicine. While some of the lectures were by obvious experts, I was left scratching my head on why others were at the podium. Meaning, some of the lectures seemed to be given by physicians or other health professionals who I felt weren’t qualified. After asking my physician colleagues who were involved in age management practices, I was told that in their opinion, A4M was a pay for play organization. This made sense to me, as it explained what I observed.
Just a few weeks ago I was invited to again speak in a section of the A4M conference on regenerative medicine. I was really hoping that in the last decade A4M had cleaned up its act. However, I was flabbergasted to see that despite the FDA having just issued very serious warning letters to many companies for selling birth tissue “stem cell” products and exosomes, the exhibit hall at A4M was filled with vendors selling these products. Meaning, in my opinion. A4M did a poor job of filtering which companies should actually be selling products and which were obviously not legal.
It Gets Worse from There…
Outside of giving my lecture and seeing a few others, I didn’t stick around at the A4M conference partly because of my past experiences and what I saw on the exhibit floor and partly because I don’t have an age management practice. Hence, I completely missed problematic lectures that were even worse than what was on the exhibit floor. That was until these pictures were posted on Linkedin:

I immediately recognized that these were from the A4M conference and I also know that both speakers are associated with Kimera (Doug Spiel left and Duncan Ross right), a company selling exosomes who had a booth at the conference. This astounded me, as I had been reassured by the physician that invited me to speak that any discussion of exosomes (which are an unapproved drug product) would be purely research-focused. I then Googled the agenda for the main conference:
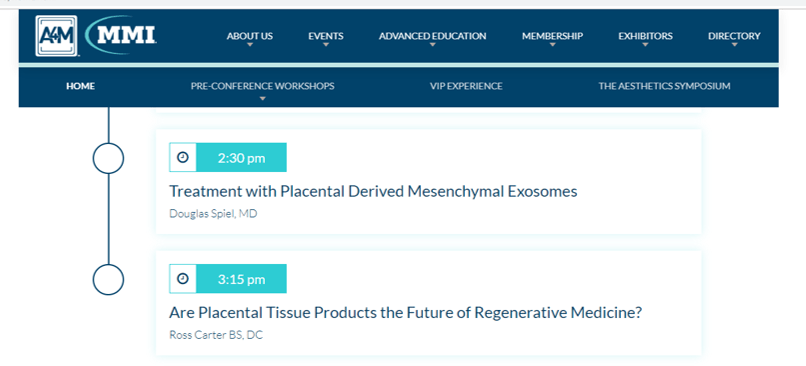
There’s Doug Spiel (on the left in the pic above) from Kimera lecturing about treatment using exosomes in patients. This is just one week after the FDA made this statement on companies selling exosomes:
“There are currently no FDA-approved exosome products. Certain clinics across the country, including some that manufacture or market violative “stem cell” products, are now also offering exosome products to patients. They deceive patients with unsubstantiated claims about the potential for these products to prevent, treat or cure various diseases or conditions. They may claim that they these products do not fall under the regulatory provisions for drugs and biological products – that is simply untrue. As a general matter, exosomes used to treat diseases and conditions in humans are regulated as drugs and biological products under the Public Health Service Act and the Federal Food Drug and Cosmetic Act and are subject to premarket review and approval requirements.”
In my opinion, there is no way to read this statement any different than exosomes are an unapproved drug product and that there are no FDA approved exosome products. Hence, allowing a speaker who worked for Kimera to lecture on the clinical uses of this exosome product while Kimera was selling its exosome products for clinical use just 100 yards away in the exhibition hall was not appropriate.
The A4M Conference Is Concerning…
Who was on the podium right after Doug Spiel? None other than this guy:

Ross Carter is a chiropractor who runs a podcast selling “stem cell” products who isn’t permitted to perform regenerative medicine procedures based on his training. He has no publications nor book chapters in this area and nobody that I know of who is an actual stem cell expert would consider Ross anything other than a salesman. Why is a chiropractor salesperson on the podium speaking to thousands of physicians on a topic where he is clearly not an expert?
I have to say I was dumbfounded by this one. At least Doug Spiel has clinical experience using exosomes and is an MD. In addition, Duncan Ross has a Ph.D. and also has basic science knowledge on the topic. The products they were selling are illegal, but at least they have advanced degrees and expertise. However, the fact that the A4M conference put a chiropractor salesperson hawking FDA non-compliant drug products on the podium is really disturbing.
The A4M Stem Cell “Fellowship”
A few years back, right after we began offering an actual one-year fellowship for young doctors to train in interventional orthobiologics, one of our actual fellows noticed that a physician was claiming to be fellowship trained by the A4M conference. I then did some research and here is the compare and contrast between what a real fellowship offers and what A4M calls a fellowship:

As you can see, what A4M offers is an extended series of pay for play courses and not a fellowship. An actual fellowship is a year (or two) where a physician works and is paid while learning an area of super-expertise. It’s thousands of hours of training, usually including requirements to publish research. Only one area is studied.
Another concerning thing about these A4M stem cell courses is that they teach in all sorts of FDA problematic stem cell treatment areas such as neurologic, cardiac, vascular, and neurodegenerative. These are all areas of patient abuse as the FDA has pointed out numerous times. In addition, they’re all areas that would require board certification to be able to care for these specific patients. For example, if you want to be able to treat neurodegenerative diseases like ALS, you should be a neurologist. No such screening occurs in the A4M “fellowship”.
Why is a series of courses called a “fellowship”? In my opinion, there’s only one reason. It’s tough to charge a physician 20 grand for a series of courses, but if you let them pretend that they can use the term “fellowship-trained” they are more likely to pony up that kind of cash.
What Is A4M’s Responsibility?
A4M was bought by Tarsus, a UK based for-profit conference company. This is from the Tarsus 2014 annual report:
“Tarsus owns and manages a portfolio of leading trade exhibitions in a range of sectors in the Emerging Markets, the USA and Europe. These exhibitions represent a vital B2B sales channel that facilitates the development and monetisation of relationships between buyers and sellers in their respective markets.”
Hence, I guess it should come as no surprise that A4M is focused on filling exhibitor halls. However, in my opinion, the problem begins when companies like Tarsus enter the medical space. Why? They attract and educate doctors who have a sacred responsibility to put their financial needs behind the patient’s medical needs. Meaning when you have a conference selling non-medical products, hooking up buyers and sellers is fine. However, when you consider physicians to be buyers, in my opinion, that creates an inherent conflict of interest.
So does have A4M have a responsibility to keep products that the FDA has clearly said aren’t legal off its agenda? Should they do more due diligence? In my opinion, they do have this responsibility. For example, at many not for profit and even for-profit conferences this next year you won’t see exosome treatment lectures because of the FDA warning. Will the A4M conference clean up its act for next year?
The upshot? In my opinion, one of the drivers of the recent explosion in the stem cell and exosome wild west is big medical conferences like A4M. A conference of this size can educate thousands of doctors in one fell swoop. Hence, regulators should pay attention to what’s happening at these big events.
If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.