Amniotic Stem Cell Injection Reviews?
I’ve been writing so much about umbilical cord “stem cell” products that I thought it was time to revisit amniotic injections. First, as a general topic of amniotic stem cell injection reviews and then specifically how Regenexx would pick an amniotic product to use. Let’s dig in.
What Is Amniotic Tissue?
Before we can understand how to perform amniotic stem cell injection reviews, I need to educate you about amniotic tissues. A baby is surrounded by a birth sac, which is filled with amniotic fluid. The sac has several layers, one of which is the amniotic membrane. These amniotic tissues (fluid and membrane) have been sold for many years for use in ophthalmology, neurosurgery, and wound healing. Then, some bright sales rep got the idea that since these tissues have a low stem cell content when surrounding a live baby, that they could be rebranded a “stem cell” product. The problem was that none of the stuff being sold has any living and functional stem cells as demonstrated by multiple research studies (1-3).
Amniotic Stem Cell Injection Reviews
If the amniotic tissue being sold has no stem cells, is there a rationale for amniotic stem cell injection reviews? While the tissue has no stem cells, there may be another reason to use it. This is because it has growth factors.
What Are Growth Factors?
Growth factors are proteins that can help or stimulate cell growth. They’re a big feature in treatments like platelet-rich plasma, which is made from concentrated blood platelets derived from the patient (4). These platelets have stores of growth factors called alpha vesicles and they release these proteins in a timed-release fashion to help a wound heal.
Fibroblast Growth Factor
A while back we became interested in amniotic products not because of fictitious claims of stem cells, but because of a growth factor called FGF (5). This acronym stands for Fibroblast Growth Factor, which stimulates the types of cells often found in tendons and ligaments. Our interest was, therefore, using it in helping heal tough to treat rotator cuff tears.
Rotator Cuff Tears
The number of physicians who are trying to practice regenerative medicine at a high level are few are far between. While there are any number of charlatans trying to bilk people out of their money by doing poor work and over-promising and under-delivering, there is also a handful of physicians who are well trained, performing high-quality work and trying to add information to the field. Among that small group, the buzz has been that certain amniotic products, while they have no stem cells, may be able to enhance the effects of PRP in tendon healing. If that’s the case, then you would want to take a look at FGF levels in these products.
Picking a Product to Use
There are many different amniotic products out there being sold by a literal army of sales reps, each claiming that theirs is the absolute best. 99.9% of the doctors would simply believe what the reps were spinning a pick a product. However, that’s NOT how we do it at Regenexx. We pursue a VERY different purchasing decision tree because we can.
Here are our criteria: The product must:
- Be Terminally sterilized – There have been far too many tragedies in the field of birth tissue sales with contaminated samples being shipped and patients being made ill. I just blogged on a new batch of contaminated umbilical cord products a few weeks back. Hence, we require the product we use to be sterilized, rather than shipped frozen to try to preserve stem cells that aren’t really there.
- Have High FGF Levels – Our goal is to use it to enhance PRP injections for tendon healing, so we want as much FGF as we can get.
- Actually Perform with Real Tendon or Stem Cells – When the product is placed in culture with real tendon cells (tenocytes) or mesenchymal stem cells, it should cause these cells to grow.
The Regnexx Experiments on Different Products
First, at Regenexx HQ in Colorado, we have a state of the art lab facility that would be at home in any university. We also have a dedicated team of scientists who work their magic there. Hence, we can test any claim that any potential vendor throws out.
Second, we know from years of testing birth tissues like umbilical cord and amniotic sourced products that some manufacturers dilute the heck out of what they produce. As an example, they take native amniotic fluid and instead of producing hundreds of samples from a single birth, they dilute that and produce thousands. We can see this in the vastly different total protein content of some products.
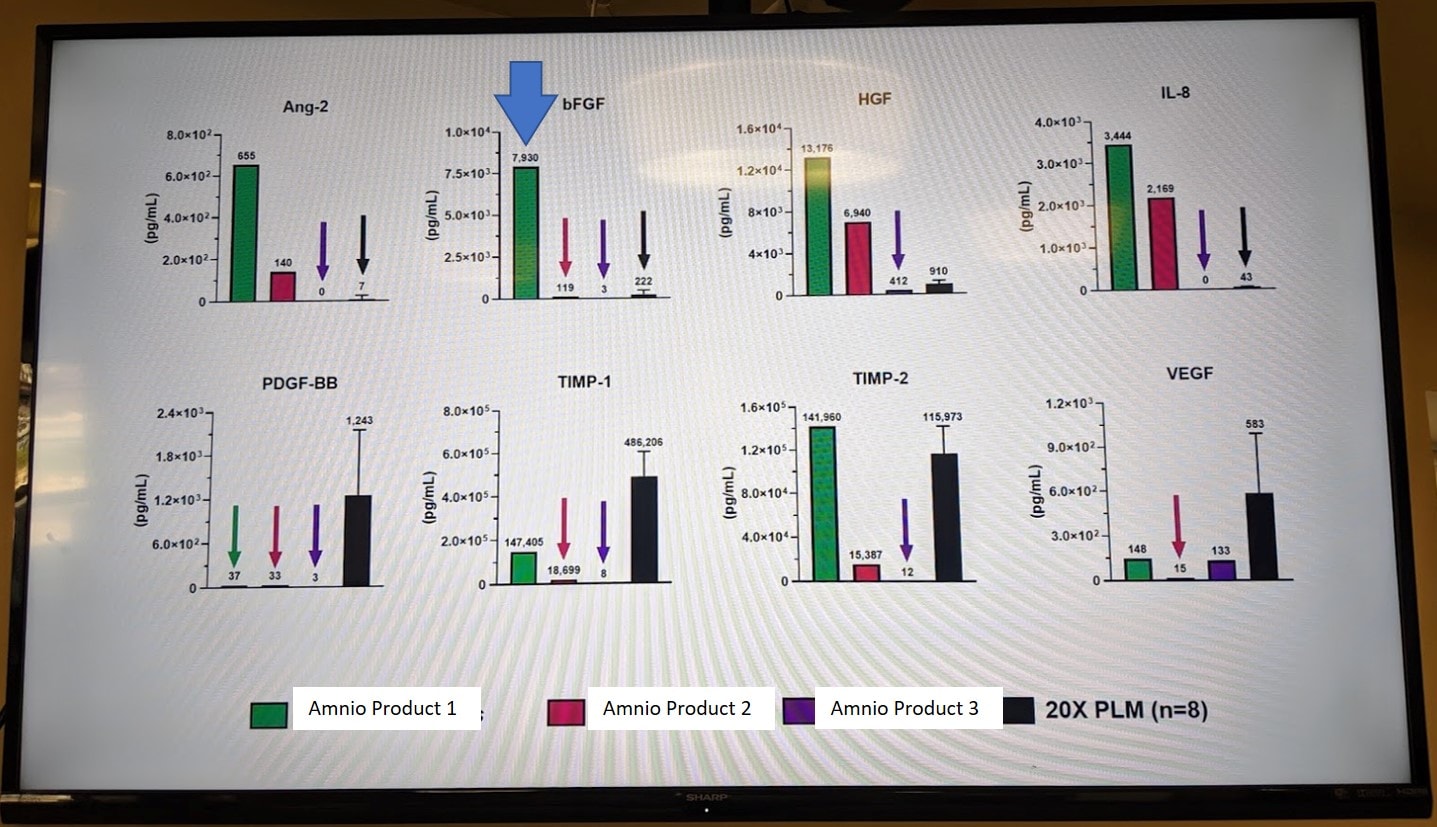
In the graphs above, we used our multiplex microarray ELISA machine to test 3 different amnio products against a platelet sample (here listed as 20X PLM). The FGF levels are listed in the second graph in the first row and the winner was “Amnio Product 1”. It beat the heck out of the other two products (likely diluted) and the platelet prep for FGF levels.
Next up was seeing if Amnio product 1 could actually perform when exposed to real tendon cells and bone marrow mesenchymal stem cells:
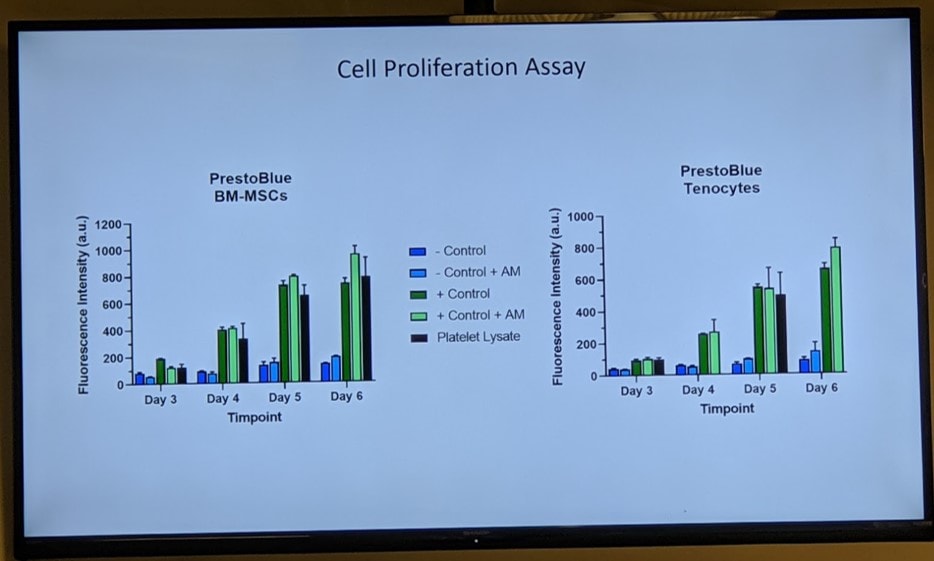
In this slide, the cell growth is being measured in culture when exposed to the amniotic product (AM). The product performed well.
The upshot? First, as you can see, the idea of amniotic stem cell injection reviews isn’t tenable. There aren’t any stem cells in these products. Second, I don’t care which doctor you visit and if that doctor is in private practice or at a major university, nobody is performing independent due diligence on the products they choose to inject like Regenexx. As you can see here, these tests cost many thousands of dollars in real lab disposables and even more in lab staff time, all to get a real read on which was the best product for our physicians to use. So when you walk into a Regenexx clinic, you know beyond a shadow of a doubt that if you get offered an amniotic product to help your tendon heal, that it’s been vetted by us, and not the sales rep!
_________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Kia C, Baldino J, Bell R, Ramji A, Uyeki C, Mazzocca A. Platelet-Rich Plasma: Review of Current Literature on its Use for Tendon and Ligament Pathology. Curr Rev Musculoskelet Med. 2018;11(4):566–572. doi: 10.1007/s12178-018-9515-y
(5) Yun YR, Won JE, Jeon E, et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010;2010:218142. Published 2010 Nov 7. doi: 10.4061/2010/218142

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
