A Big Conference a Week After the Public FDA Warning Letters
Last week the FDA released multiple warning letters and public announcements that made it crystal clear that companies selling exosome and umbilical cord “stem cell” products were violating the law. This week I was asked to lecture in a regenerative medicine section of the A4M conference in Las Vegas. Hence, I took a stroll through the exhibitor space to see who had modified their messaging to come into compliance. The results blew me away.
Prior FDA Letters to Birth Tissue Vendors
The FDA to date has issued many warning and other letters to various birth tissue manufacturers. These all generally say that they are not permitted to call these stem cell products using a basic donor tissue registration. These have included letters to:
To explain further, the issue with these companies from an FDA standpoint is what they claim to be selling. Remember that the FDA is a system whereby what you claim to sell drives the regulatory paradigm. For example, charcoal, when sold for water purification, doesn’t need FDA approval, but when it’s sold to treat poisoning, it does need FDA approval. Hence, as we and others have shown, while none of these companies actually sell products that contain live and viable stem cells, just their claim to sell stem cells requires that they get full FDA approval and not a simple tissue registration.
The Recent FDA Warning
While anyone reading the above letters would be very clear that they are not permitted to sell a birth tissue product and claim that it has stem cells, believe it or not, few seemed to get the memo. Hence, the FDA did something they rarely do this past week-they went big and bold by issuing two different public warnings. One was about Liveyon and two other manufacturers of umbilical cord tissue that were claiming to sell stem cell products. The other was a shot across the bow to companies claiming to sell exosome products. Both letters made it very clear to all of these companies that you had better have a full FDA drug approval in place if you claim to sell either umbilical cord derived stem cells or exosomes. They also made it clear that NONE of these companies making these claims has FDA approval.
My A4M Experience
Given that the above warning letters were a really big deal, I expected these to put a lid on anyone who was claiming to sell umbilical cord stem cells or exosomes. Hence, while I had been asked to give a lecture at the annual A4M conference in Las Vegas on orthopedic regenerative medicine on our randomized controlled trials, I was blown away that very few physicians understood the significance of these letters. For example, when I asked a lecturer on exosomes about the FDA warning that he failed to mention during his lecture, it seemed like I was bringing up the fact that the dog had just farted. Nobody seemed to want to talk frankly about it. That didn’t stop me from giving a barn burner of a lecture when I was on the podium. I very clearly told the room full of physicians all about the fact that anybody in the exhibition hall that claimed to be selling stem cells or exosomes was, in my opinion, violating federal law.
The Liveyon Response
Interestingly, Liveyon announced on Friday that it would cease sales of its product, pending a future FDA drug approval. I’m not sure that there could be any move that more clearly demonstrates that the FDA means business here. So how did the companies exhibiting their products at the massive A4M conference react?
The A4M Exhibitor Space
First, the exhibitor hall for this huge conference is massive. It took the better part of an hour to walk the whole thing. I counted 7 companies that were selling either umbilical cord tissue or exosomes (pictures above). These were:
- Predictive Biotech-Wharton’s Jelly
- Kimera-Exosomes
- Organicell-Exosomes
- Invitrx-Umbilical cord “stem cells”
- Regenerative Labs-Wharton’s Jelly “stem cells”
- XO Stem-Exosomes
- Direct Biologics-Exosomes
I have Predictive Biotech listed first, as I have covered them many times on the blog and I was surprised to see that their booth didn’t use the term “stem cells”. They have never been shy about advertising that one of their products, “CoreCyte” is a stem cell product, so I checked their website. Have they changed their ways online? Nope. A check of their website on 12/13 demonstrated the same table that’s been there for a while now still claims that CoreCyte is a viable stem cell product.
Kimera has been the biggest exosome player in the market, so it’s not surprising that their booth had the term “exosomes” plastered all over it. Their website also states that they still sell exosomes. So in my opinion, Kimera is still violating FDA regulations.
Here’s a summary of all of the companies and what I found:
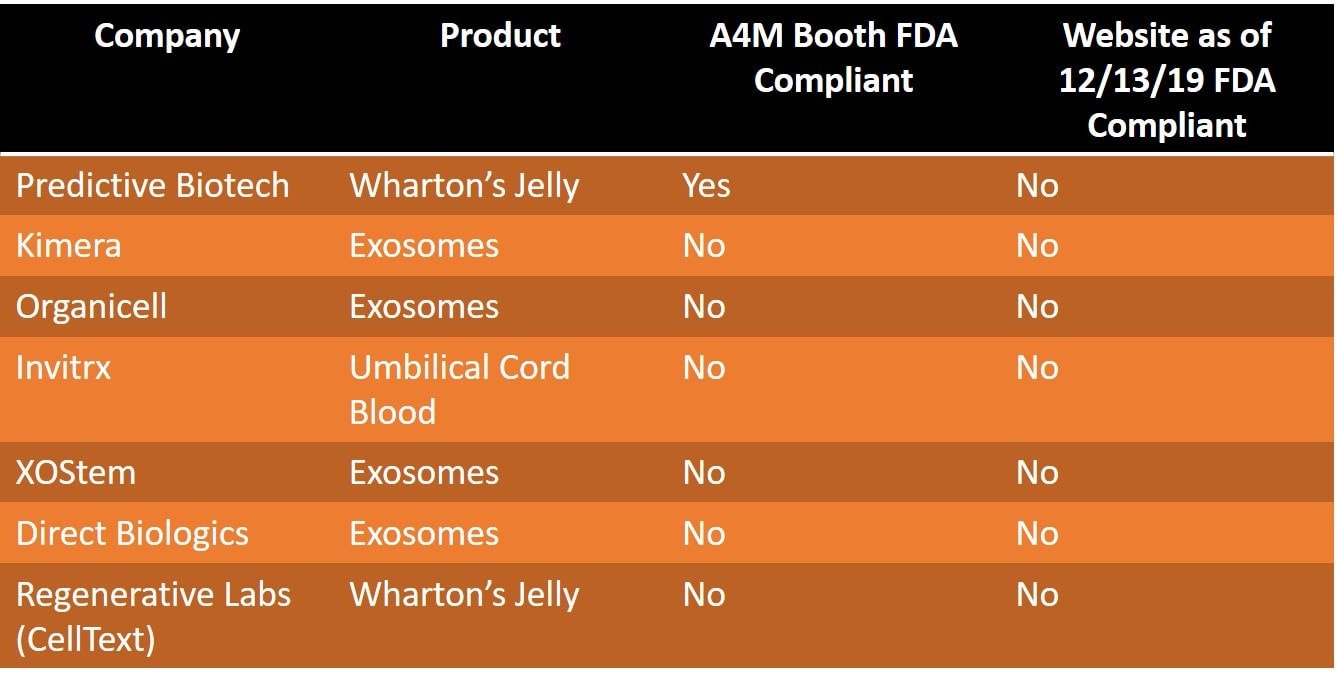
As you can see, the other companies including Organicell, Invitrx, XOStem, Direct Biologics, and Regenerative Labs, in my opinion, were all not FDA compliant either in their exhibitor booth or on their websites. So that’s 100% non-compliance even after a very big FDA public warning.
What Will Happen Next?
Is there a way that the FDA can get these companies to comply? That seems to be the billion-dollar question. More warning letters? More public warnings? Criminal charges? Your guess is as good as mine.
The upshot? A week after the FDA fired a serious shot across the bow of the birth tissue and exosome industry, these companies are still selling unapproved drug products. Will these companies stop selling these products or will it take enhanced FDA enforcement? Only time will tell.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.