How Doing It Right Can Go Wrong… The DynaCord Story

You work for years to create a true drug product with consistent potency and purity. You work hard to get an FDA Masterfile and finally begin selling your product for lab use only. Then one day you see your product being sold for clinical use in patients and you gasp because you know that this is illegal. You then issue a raft of cease and desist letters, but that has little impact. That’s the DynaCord story. Let’s dig in.
What are Exosomes?
Exosomes are little vesicles released by cells that have a payload that instructs another cell to do something. The idea of using them for therapy is that they may help us to heal damage. However, we don’t yet possess the knowledge at this point to sift through the hundreds of messages inside these vesicles to find the ones that optimize healing. To learn more, watch my video below:
However, there is one approach that would work. You create a consistent product that contains the same type of exosome messages that can be replicated over and over through a strict manufacturing process. You then test that in the lab to determine if it can do things like improve the ability of fibroblasts to close a gap in a scratch test. You then apply to the FDA for an IND to clinically test the product in wound care and if all goes well after clinical trials are completed, your product gets a BLA.
That’s basically where the real DynaCord story begins. However, then there’s a fake DyanCord story. Huh? Let’s dive deeper.
DynaCord
This story has lots of twists and turns like any good story. At first, I believed this was just another scam product. Then I wasn’t so sure. Then I was certain. Finally, I learned the truth about a company trying hard to do it right, but that’s getting abused by sales organizations claiming to sell its product.
It begins with a sales rep posting a suggestion on a Linkedin post that we physicians should try DynaCord. What’s that?
After some digging, I found another Internet post. I noticed that fellow orthobiologics pioneer and stalwart of what’s right and wrong about our field, Don Bufford had been approached a few months ago to purchase DynaCord:
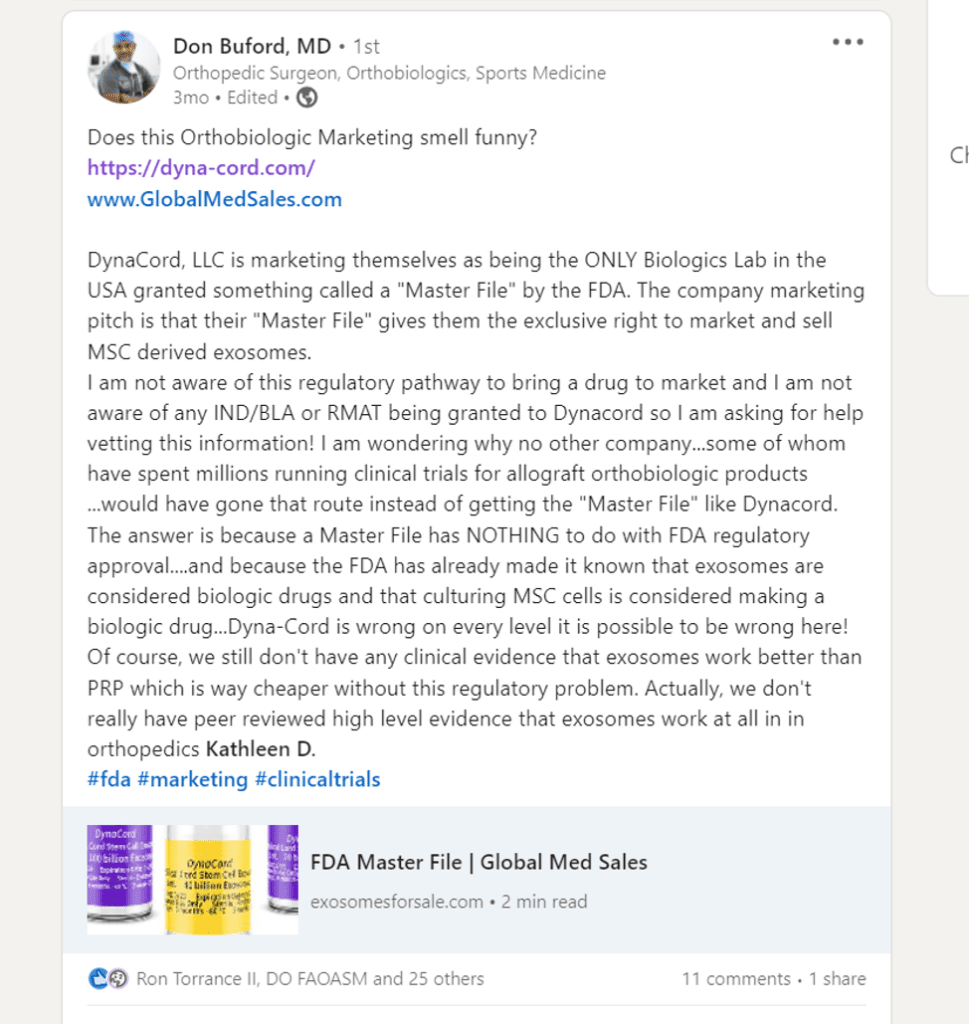
A Google search found that DynaCord is a company that sells exosomes:

On their website they have three products:
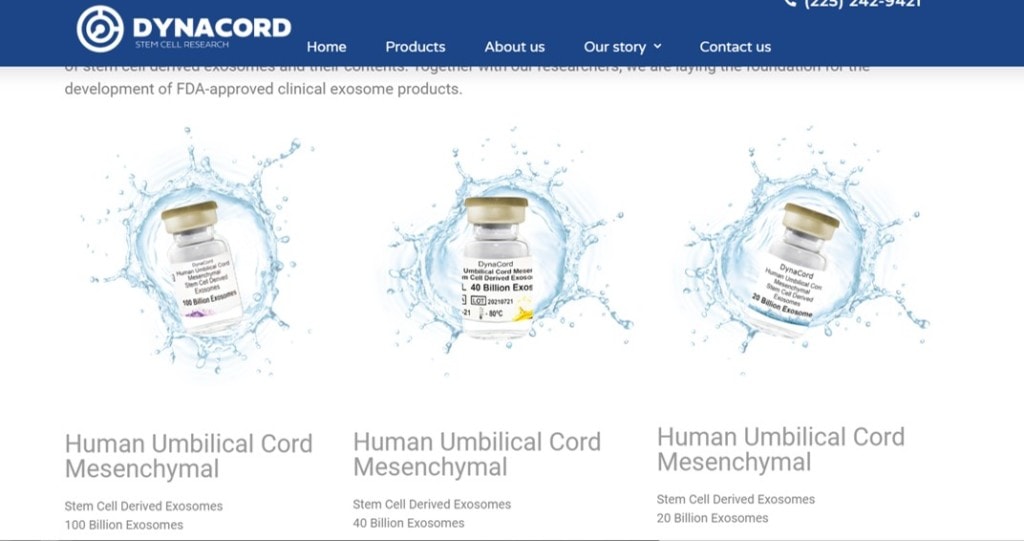
They claim to have an FDA “Masterfile”:

That means they have worked with the FDA to treat their exosome product like a drug. Meaning they use a strict manufacturing process that produces a consistent product time and time again. All good, right?
Reading further on the DynaCord website, they make it clear that their product is for sale only for research purposes. Again, that sounds right. They don’t have full approval to sell the product for clinical use, but they can sell it to labs for in-vitro research and to companies that have an FDA IND (approval) to perform clinical research tied back to this product.
NorthEast Medical Supply
So why would some random sales rep post that I should use DynaCord in my patients? After all, it seems like the company has a strict policy not to sell this stuff to anyone who might use it in patients outside of an FDA-approved study. Like anything these days, the answer was only another Google search away.
It didn’t take long to find a company selling DynaCord to doctors, Northeast Medical Supply. They had a fancy website claiming that I could use DynaCrod for injection and infusion. I then called that number and eventually spoke to a salesperson who said that while DynaCord was for research use only, I could purchase it as a physician and use it as I saw fit. I asked about orthopedic use and was told that there would be no issues and that the sales rep had physician clients that were using it to treat everything from Erectile Dysfunction to hair loss to joints. Case closed on DynaCord, right? Just another “claim that you’re selling it for research and then “wink-wink” really sell it to doctors who you know will use it clinically” scam. Right? Wrong.
DynaCord 2.0
I had also called DynaCord the actual company as well and it wasn’t long before I heard back from them. What I found out was that the company is trying to do it all the right way. They have a master file and they have applied for an IND in wound care and would only sell me the product if I were a research lab or a company that had gotten FDA approval to use their product in a clinical trial (IND). Then who the heck did I just speak to? The guy who was hard selling me on buying DynaCord “for research use only” but then really using it to inject into my patients? That guy apparently is part of a company that has received cease and desist letters from DynaCord and who has been told to stop trying to sell their product to doctors. Turns out that DynaCord did hire sales reps to sell its product to labs, but somehow medical sales reps got ahold of it and are selling it to doctors, which is something they have been trying hard to clean up, but it’s like playing Whac-A-Mole. Meaning that DynaCord wants to make sure that nobody uses their product outside of an FDA-approved clinical trial until they get full FDA approval, which is years away.
The Cellularity Story
I’ve blogged before on a legit company that’s trying to do things right called Cellularity which had rouge sales reps trying to sell its products for orthopedic uses and claiming that Medicare would reimburse you. They also have sent countless cease and desist letters and played Wac-A-Mole. They also have products as part of real FDA INDs and clinical trials.
Why is this a Thing with Exosomes? The FDA and Exosomes
The FDA prohibits the sale of any commercial product claiming to have stem cells without extensive clinical trials and product approval. That can take 5-10 years and cost hundreds of millions of dollars. To get around that, some companies began to sell “exosomes” produced by stem cells, on the shaky theory that the FDA would allow this product to be sold. They didn’t get FDA approval, but instead, that all went “poof” when the agency put out this 2019 consumer warning:
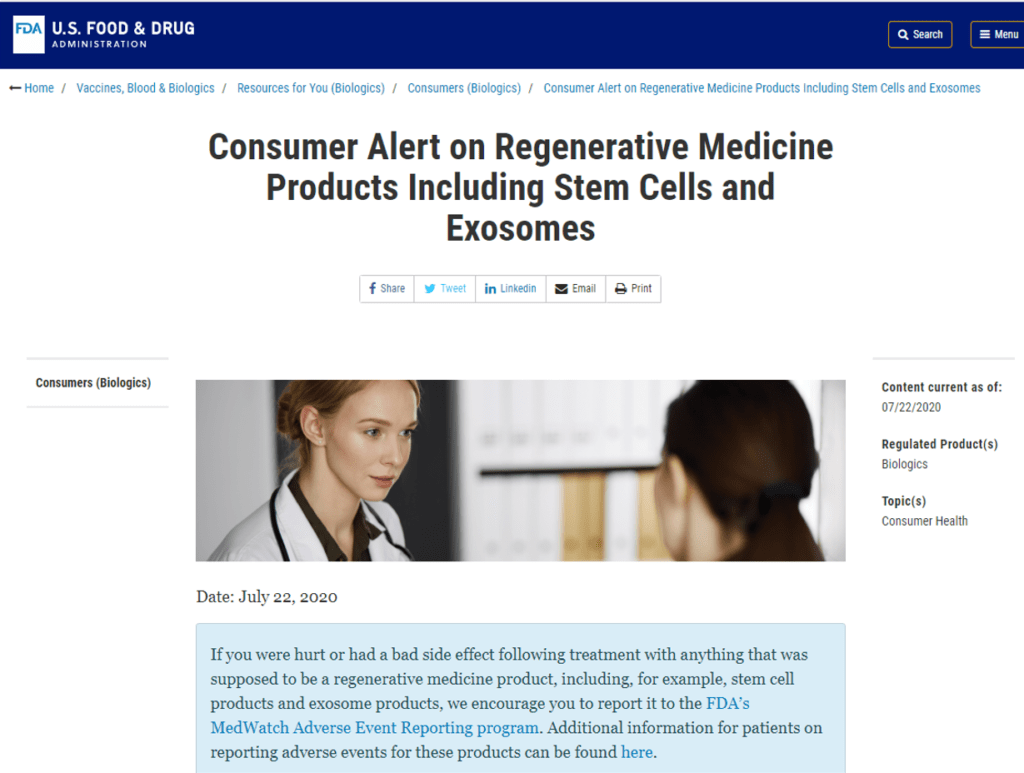
What does the FDA say? It’s illegal to sell exosomes for clinical use. Despite that, many companies have sold exosomes and if you look on Facebook, they’re still “a thing”. So while the FDA says this is illegal, it’s being done. The FDA has also sent many letters to manufacturers, but this is also like playing “Whac-A-Mole”.
The upshot? Even companies working hard to comply with FDA regulations and create new drugs are having trouble with the massive army of sales reps who are still pushing magic products without FDA approval. If you as a patient or physician are offered DynaCord, realize that it’s an unapproved and illegal drug product until its makers are able to get through the FDA’s clinical trial process. Also, realize that its makers don’t want you using it, despite what the medical sales reps claim.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.