What Is Evolution Biologyx? What Do They Have to Do with Interfyl?

Credit: Shutterstock
It’s a full-time job keeping up with the shenanigans of sales reps selling birth tissues. This one is interesting because it involves a very legit billion-dollar company and an outfit that claims to be selling their product. Add in a heavy helping of high-risk Medicare billing ideas and we have a twisted tale. Let’s dig in.
The History of Birth Tissue Scams
If you’re an avid reader of this blog, it would be reasonable to wonder why I’ve spent so much time on this topic. The reason is that this field has always had two main camps. One that has been working hard to do it right and another that always focused on maximizing the financial opportunity at the expense of patients. Bench scientists in this field have often been unable to see that dichotomy due to their own biases, so exposing and defining that divide has become a key mission of this blog.
My first exposure to the dark side of the birth tissues industry goes back to about 2014, so it’s amazing that this industry has been scamming doctors and patients for almost a decade now. However, realize that for every instance of bad there’s also good. Hence, there are hard-working companies in the birth tissues industry trying to do things right.
Back then a company called BioD was telling sales reps that it was selling a vial of amniotic fluid that contained loads of stem cells. As you know from reading this blog, we tested several amniotic products in our advanced lab and found no living cells, let alone stem cells. That took a few years to get out there as did the results of other scientists who tested these products, but eventually, the sales reps for amniotic companies let that myth die (1-3). Then came the concept that these amniotic products were growth factor rich, which is a myth that still survives to this day. When we tested that one, it was only partially true.
Around about 3-4 years ago, the amniotic space was pretty battered by all the revelations, so a new product line became available: umbilical cord tissue. This began with umbilical cord blood and then morphed into the Wharton’s Jelly. All of a sudden the scam that these were products were rich in live and functional stem cells reared its head yet again! As John Lovit used to say, “Yeah…That’s the ticket!” We tested these umbilical cord products as well and found that this claim was also fiction (4). Then the umbilical cord blood products industry caved when the Liveyon contamination occurred. However, new Wharton’s Jelly stem cell scams took the place of cord blood and continue to this day.
The next scam began as a way to resurrect amniotic tissue. Again, after our information got out that these products contained no stem cells and the competition from Wharton’s Jelly began to sink in, that industry needed a way to refresh itself. That lead to the Q-code scam. This one was pretty ingenious, as the companies found consultants who snuck in all sorts of language about non-FDA cleared clinical indictions into their product code descriptions and then found shady billing companies to get Medicare to pay those claims for orthopedic and spine injections. The Wharton’s Jelly manufacturers were now competing against a juggernaut, so they also tried to do the same thing and a few made it through before Peter Marks of FDA put a stop to all such approvals and began rescinding the billing codes that were approved by CMS. In addition, now that the providers who billed this stuff are getting Civil Investigative Demands by the justice department, that put a damper on sales.
An Email to a Colleague
Two different colleagues reached out to me (unrelated to each other) on this one. This is the email sent to one of them:
“From: Eric Tuscan <[email protected]>
Date: Wednesday, August 4 [time redacted]
Subject: New FDA designated 361 product with HCPCS code
Hi Dr. [name redacted],
We have not spoken in a couple years so I hope you are well. I am reaching out to share with you a new HCTP that has FDA RFD designation (FDA file 2004.046) as a 361 product for injection into damaged or inadequate connective tissue. I’m sure you are aware of the changes in the 361 space since 5/31/21 and the new enforcement requirement to have premarket approval, our product Interfyl has that and a matching CMS HCPS code (Q4171). As you can see from the FDA link below companies can’t just state they are compliant like in the past. Attached and below is information and a case study for your review.
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/questions-and-answers-regarding-end-compliance-and-enforcement-policy-certain-human-cells-tissues-or
Let me know when you have a few minutes to discuss.
Regards,
Eric Tuscan
National Sales-Evolution Biologyx”
What Is Evolution Biologyx?
Based on an FDA Tissue Establishment look-up, Evolution Biologyx is not the manufacturer of Interfyl, Instead, it’s a marketing operation reselling the product. What’s Interfyl? This is from their website:
“Interfyl® is a decellularized human placental connective tissue matrix (CTM) to be used for the replacement or supplementation of damaged or inadequate integumental tissue…Please reference Medicare Q Code Q4171 for Interfyl Human Placental Connective Tissue Matrix.”
There’s a couple of interesting things here. First is the Q-code, which we’ll revisit below. However, on the website is a legit company called Celularity mentioned as the manufacturer of Interfyl. They’re a billion-dollar, publically traded entity. More on that below.
Billing Medicare?
The email above makes it very clear that there is a Medicare billing code for Interfyl. That by itself raises no red flags, as Medicare covers these types of products for specific clinical scenarios in wound care. That means that if an elderly patient develops a bedsore that can’t be healed in other ways, you can use a birth tissue product to help that wound heal. However, where the problem begins is that there is NO COVERAGE for any orthopedic indication. Hence, if you use this stuff to inject a knee, hip, shoulder, or spine, there is NO COVERAGE.
Next, my colleague attended a seminar put on by the company on how to bill Medicare. This slide came from that presentation:
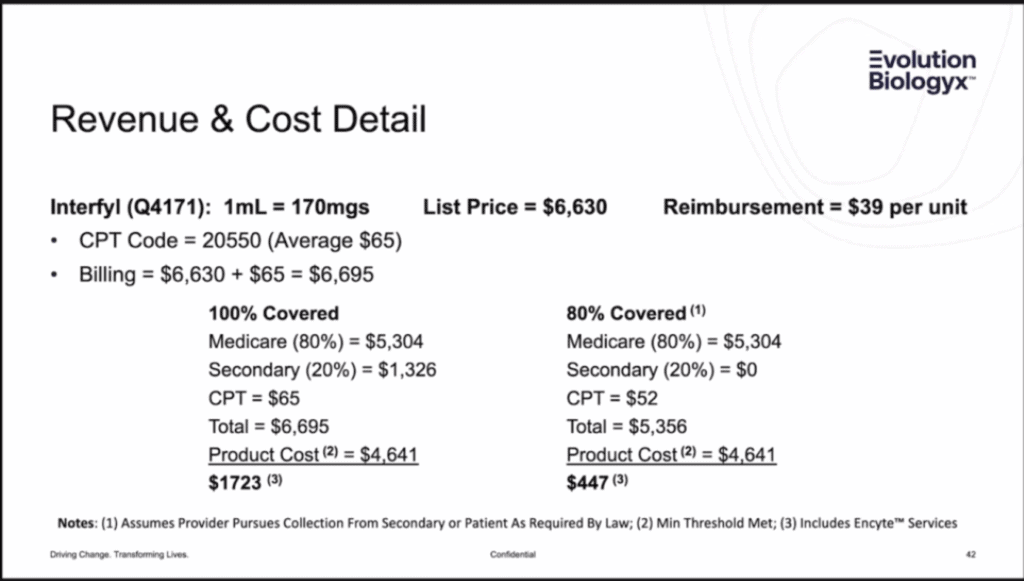
Here we see a pricing structure similar to the one other companies are featuring. Medicare pays X, you purchase the product at a discount, and you keep the difference. From speaking to healthcare attorneys, while there are scenarios where this may be legal, that’s for drugs used for clinical indications covered under a Medicare guideline (LCD). The goal of that safe harbor is to move procedures from more expensive hospitals into doctors’ offices by offering an incentive. However, what if the product isn’t covered for that purpose? Then you’re not saving Medicare any money because it’s not used in hospitals. That’s likely why we see a recent law blog state that the Justice Department is currently investigating birth tissue companies and physicians who used these rebate agreements to bill Medicare for birth tissues used in Orthopedics.
The physician that attended this webinar said that while they discussed legally billing for wound care, the company told physicians that if this product is billed “to fill a defect” in an orthopedic setting (i.e. a cartilage defect in a knee), then it could be billed to Medicare. They also stated that another use could be muscle trigger point injections. However, neither of these is a covered indication in any Medicare guideline. Again, crossing that line from wound care into orthopedic use.
I called the sales rep that sent the email, Eric Tuscan, but he would tell me very little. He did say that they weren’t sure Medicare would pay for orthopedic injections, but according to my source who attended the webinar, they are encouraging physicians to bill it that way to Medicare.
Celularity?
It was surprising to see Celularity mentioned on the Evolution Biologyx website. Why? While Evolution Biologyx is a sales outfit, Celularity is a real cellular drug research corporation with a billion-dollar market cap. They are working with FDA to get cell drugs approved and I have never seen them get mixed up in anything that isn’t 100% legit.
Since I know some of the people who run this company, I reached out to a board member who put me in touch with high-level management. After I explained my concerns. I got an earful! Meaning they were NOT happy.
Why? Cellularity just signed an exclusive distribution agreement for Interfyl with Arthrex earlier this month. That agreement provides an exclusive to Arthrex (one of the world’s largest orthopedic equipment companies) for orthopedic use. My high-level contact in Cellularity who oversees this part of their business confirmed that Evolution Biologyx is not permitted to sell Interfyl for orthopedic use, but only for wound care. Hence, for Evolution to send a marketing email to a physician that has no involvement in wound care but only treats musculoskeletal conditions is a problem.
He made VERY CLEAR that Celularity also wants no part of any effort to bill Medicare for orthopedic injections. The company has a zero-tolerance policy for creative Medicare billing.
What is Evolution Biologyx allowed to do with Interfyl? They can sell it for wound care applications ONLY. My Celularity contact stated that they have literally sent out half a dozen cease and desist letters in the past week to rouge sales reps claiming to sell their product. In this case, we have a company (Evolution) that is allowed to sell the product for wound care (a very legitimate use), but no authorization to sell it for orthopedics and certainly no approval to promote Medicare billing for that clinical indication.
Rogue Sales Reps
These last two blogs cover an interesting trend. The companies that make this stuff often use sales reps as independent contractors who are then paid a commission for the sale. Add in that many birth tissue manufacturers dumped inventories when the FDA crackdown happened on May, 31st of this year, and you now have sales reps in charge of marketing and selling product that is no longer manufactured. It seems that many are getting very creative in how they move that product.
What responsibility does a birth tissue manufacturer have that hires an independent sales rep? Certainly, if the company contracts with the rep it bears full responsibility for what that rep communicates about that product and has to have strict compliance programs in place to keep the rep on the same page as the company. However, what about a company that dumps product, leaves this space, and has reps doing things they shouldn’t with its branded product?
The upshot? The Evolution Biologyx story took a few days to investigate, but in the end, this turned out to be a reseller that isn’t permitted to sell the product for orthopedic use, nor are they licensed by Celularity to bill Medicare for orthopedic indications. Hence, I suspect that this one will take care of itself! However, there is still the bigger issue of sales reps engaging in marketing that isn’t FDA, FTC, or CMS complaint. That’s a new problem for regulators.
[Update: Sept 14th, 2021-I was notified that Evolution Biologyx took immediate action because the sales rep involved was not following their internal policies. I have been informed by a third party that they terminated their relationship with that sales rep.]
___________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Panero AJ, Hirahara AM, Andersen WJ, Rothenberg J, Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. Am J Sports Med. 2019 Apr;47(5):1230-1235. doi: 10.1177/0363546519829034. Epub 2019 Mar 7. PMID: 30844295.
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Aug 16:3635465211031275. doi: 10.1177/03635465211031275. Epub ahead of print. PMID: 34398643.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
