Our Latest Paper on Umbilical Cord “Stem Cell” Products Gets Published!

One of the staples of the “Stem Cell Wild West” has been tissue manufacturers claiming that they’re selling Umbilical Cord stem cell products. Based on our lab testing of many products we knew this was fiction, but that also needed to be peer-reviewed and published. That just happened. Let’s dig in.
Umbilical Cord “Stem Cells”
If you take an umbilical cord from a live birth and walk that cord to a research lab, they will be able to get mesenchymal stem cells to grow out of the Wharton’s Jelly in the cord. The problem starts when you store that cord in the hospital, that’s then transported to a processing facility, then it’s stored until the processors can work on it, that then is processed and the Wharton’s Jelly extracted and isolated, then placed in vails, then frozen to -80C, then stored, then shipped across the country, and then stored, and then finally shock thawed by the doctor. By the time the cells have gone through this Dante’s Inferno, they are dead or dying.
While all of this would be obvious to anyone who has worked with live stem cells, isolation, culture expansion, and cryopreservation, it’s not obvious to most physicians who have no experience in this area. Hence, manufacturers and sales reps are able to get physicians to bite on the story that their vials containing Wharton’s Jelly have millions of live and highly functional stem cells. Therein begins the problem, as physicians and providers then pass that myth on to patients.
My History with Birth Tissue Stem Cells
I do have experience with culture-expanding and freezing mesenchymal stem cells (MSCs). Regenexx also has a state-of-the-art university-equipped lab with actual scientists who can test claims like, “This vial contains millions of live MSCs”. Way back when in 2014 when I first heard that story of live stem cells our scientists tested the product making that claim. It had ZERO living MSCs. Then we tested a few other birth tissue products that also had no living stem cells. At that point our interest was piqued, so we tested more amniotic products and presented that data at a medical conference (1):
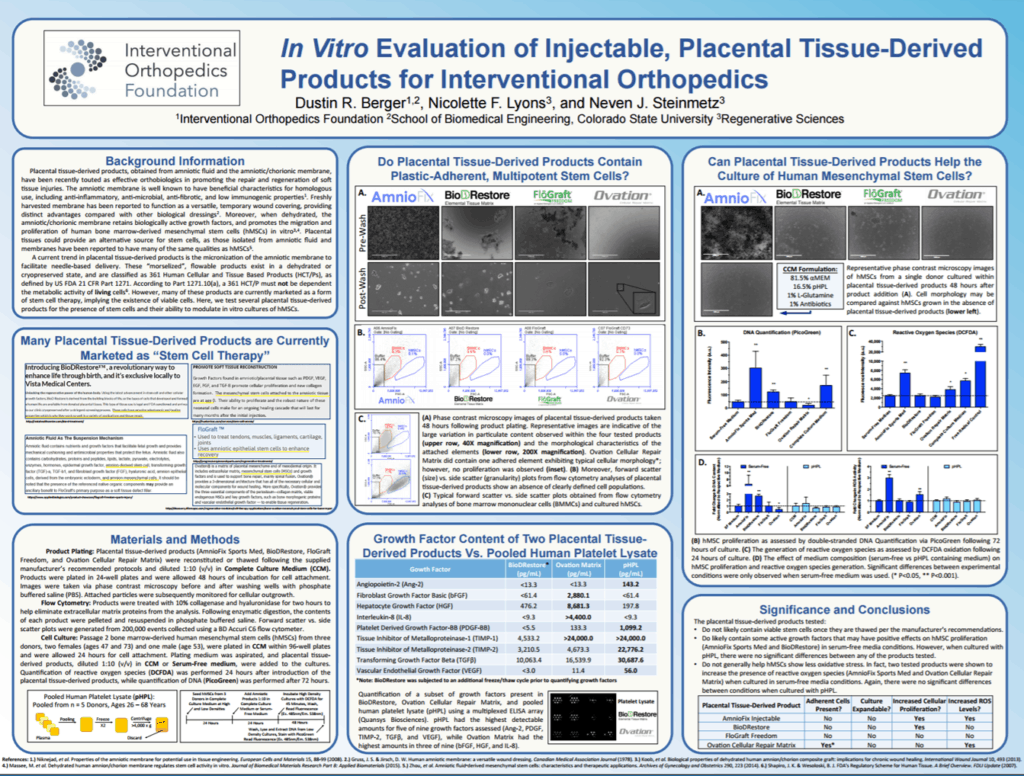
We again found no living and viable stem cells. Then the birth tissues industry got wise to our and the findings of Panero at UC Davis (2) and Fortier at Cornell (3) who also tested these products and found the same thing. Hence, the industry shifted its focus from Amniotic to Umbilical Cord products. At first, this was umbilical cord blood, but then it quickly morphed to the Wharton’s Jelly found inside the umbilical cords. So the new story became, “well maybe Amniotic products don’t have stem cells, but Umbilical Cord products do have stem cells’. Our lab testing again showed no stem cells, so it was time to publish a paper on all of this insanity. This launched our most recent publication which was done in conjunction with the CSU Translational Medicine Institute.
The New Research
Our new paper was just published in the American Journal of Sports Medicine this week (4). Our team, working in conjunction with CSU, tested 5 Umbilical Cord products made by companies that all claimed that these products had live and functional mesenchymal stem cells (CharaCore, Liveyon PurePro, Richgen, Stemell, and StemVive). As a control, we used Bone Marrow Concentrate taken from elderly patients. Our scientists thawed these products per the manufacturer’s recommendations and then looked at:
- Post thaw viability
- CFU-f
- Proteomics
Post-thaw Viability
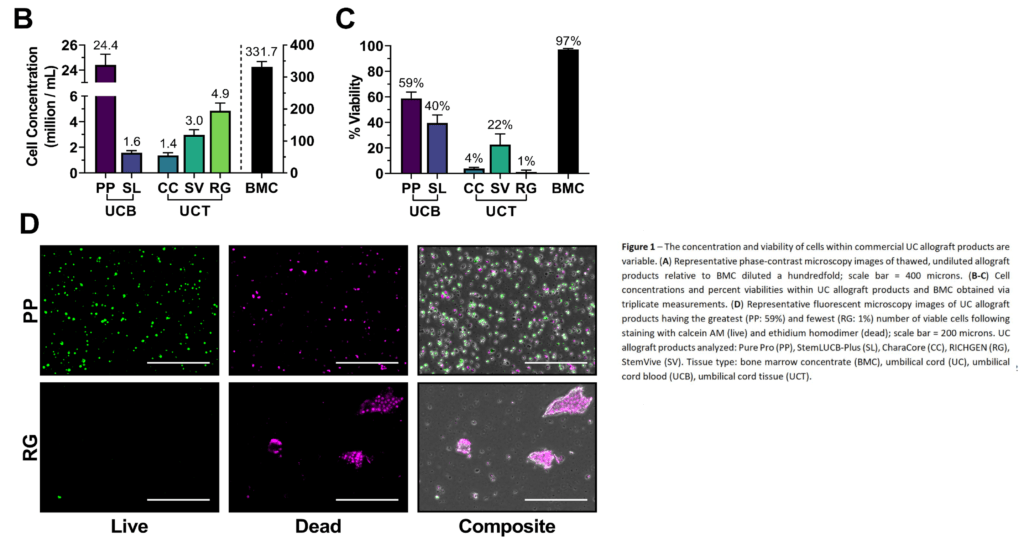
When you’re thawing frozen cells there’s a critical rule – thaw them very slowly. Why? Quick thawing can kill cells. However, all manufacturers recommended a fast thaw and not the controlled rate thaw that would be used by a research lab. Why? Doctors and other medical providers don’t have a lab, so usually, this stuff is warmed on a tray at room temperature or in someone’s hand.
After cells are thawed, you can measure the number that are living or dead using various stains and this will give you some idea of the health of the cell population. So how did these products do? First, Bone Marrow Concentrate from the elderly was 97% viable, which is pretty typical for these cells and means very healthy cells. However, the best Umbilical Cord product scored a 59% (Liveyon PurePro), which is not great. That basically means the cell population is not healthy. However, viability went way down from there with the other products clocking in at 40%, 22%, 4%, and 1%. Meaning these cells were DOA. See the graph above.
CFU-f
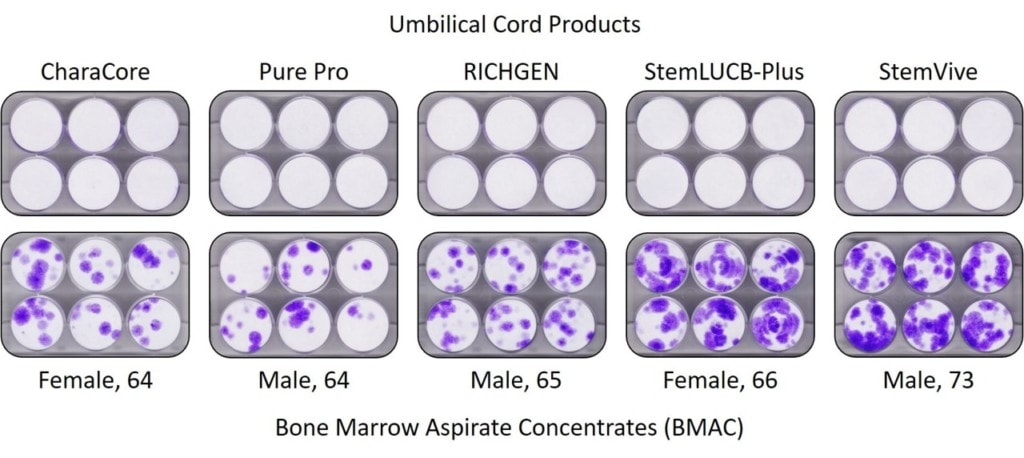
Next up is a CFU-f test. What’s that? Here we plated the cell products and bone marrow and then placed these in culture. The goal of this test is to get the mesenchymal stem cells to adhere to the plates and grow. After that has happened, the cell colonies are stained and if present will show up as purple dots like you see above in all the elderly BMAC samples on the bottom row. What you don’t see is any stem cell colonies in any of the Umbilical Cord products in the top row. So bone marrow from elderly people has MANY MORE stem cells than Umbilical Cord products, which have NONE.
The results of this test are significant as the companies making these products and the providers using them have literally given thousands of Seminars to the elderly telling them that their bodies have far too few stem cells and thus that’s why they need to use umbilical cord products, which have millions of young, vital, and healthy stem cells. This test above shows that the truth is actually the opposite. Again, the bone marrow of elderly people has lots of stem cells while these products have NONE.
Proteomics
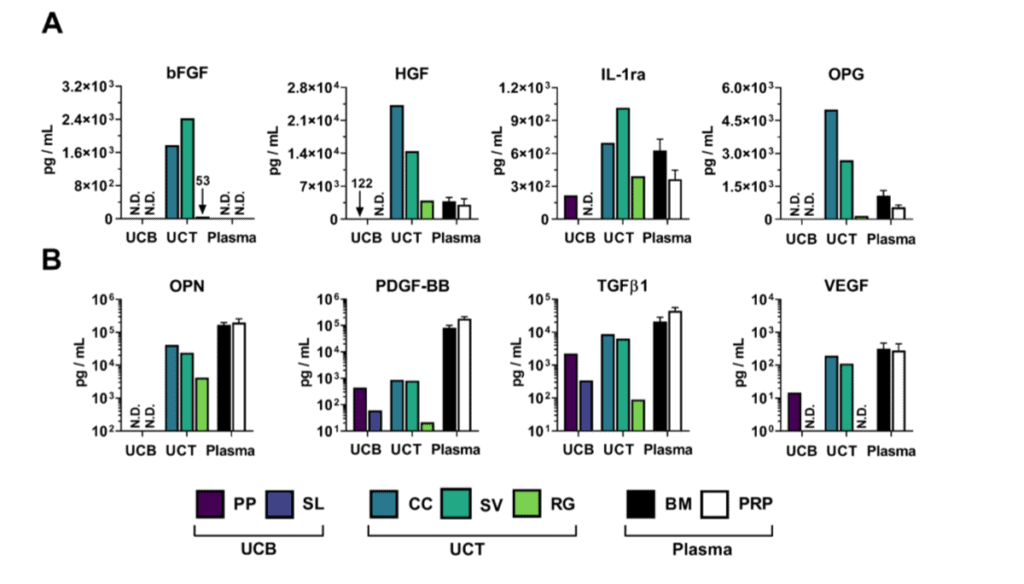
Proteomics is a fancy research term that means identifying and measuring lots of different proteins in a sample. In this case, these are growth factors and cytokines that help lots of different things grow, reduce inflammation, or stoke up inflammation. The good news is that we identified hundreds of growth factors and cytokines in both Umbilical Cord products and elderly Bone Marrow Concentrate. In some cases, there were higher levels of one over the other and in other cases vice versa.
As an example, Wharton’s Jelly had higher levels of b-FGF, a growth factor that can help tendon’s heal. Now that’s also a bit deceiving as the live stem cells in Bone Marrow Concentrate will also pump that stuff out when they detect it’s needed, which doesn’t happen in the umbilical product because the stem cells are dead. In another case, the anti-inflammatory cytokine IL-1ra was a bit higher in the WJ product than bone marrow, which is a good thing that could cause a short-term reduction in swelling in something like an arthritic knee. On the other hand, bone marrow had a higher level of TGF-b, a critical growth factor for cartilage repair. So the proteomics comparison was pretty much a wash between Umbilical Cord products and bone marrow, with the bone marrow obviously having live stem cells that can produce more growth factors and cytokines as needed and the Umbilical Cord products no living stem cells. Meaning, what you see is what you get in the Umbilical Cord products.
The Scams Continue
Somewhere in the US this week, likely thousands of patients are being told that they’re getting an Umbilical Cord stem cell treatment. As you can see above, this is fiction, and now that all of these scientists have published their data and we have published ours, outright consumer fraud. Why is this still happening? Who wants to pay $10,000 to get both of their knees squirted with Wharton’s Jelly? However, the elderly will pay that much if they believe that they’re getting millions of young stem cells injected into their knee.
The upshot? It’s time to end the farce that Umbilical Cord products have loads of stem cells. If you’re elderly, your own bone marrow has lots of stem cells while these products being hawked at Seminars at the local steakhouse have none. Hopefully, our research and that published by other scientists can be used by state attorney generals, the FDA, and FTC to put these scammers behind bars.
_______________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Panero AJ, Hirahara AM, Andersen WJ, Rothenberg J, Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. Am J Sports Med. 2019 Apr;47(5):1230-1235. doi: 10.1177/0363546519829034. Epub 2019 Mar 7. PMID: 30844295.
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Aug 16:3635465211031275. doi: 10.1177/03635465211031275. Epub ahead of print. PMID: 34398643.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
