Regenerative Medical Supply: Scam Alert
There isn’t a week that goes by that I don’t hear about another amniotic, umbilical cord, or exosome scam. The latest is interesting in that the sales rep tried to poach one of our most loyal patients on Facebook. How that was done is educational in that the sales pitch contained multiple easily debunkable falsehoods. Let’s dive in.
A Facebook Message
During a busy last week, I got a text from a longtime patient that she had been poached on Facebook. She apparently left a supportive message on our Facebook page and soon after, a sales rep for a medical distributor sent her this message:
“Hi Donna, I saw your interest in the Regenexx ad. Just to let you know, Stem cells are covered by insurance through a different company. Regenexx does not have FDA clearance and insurance approval like we do. Our company has invested time and money over the last five years to be the only stem cell product on the market that IS covered by Medicare and most Insurance now. Tricare also covers us, but is covered at 100%. It is approved for ALL joints. Please talk to your Dr about us and share that link below with them. Our product is one your primary care Dr. can use to regenerate your own cartilage with a simple 15 minute injection.
Lenora Hughes
Biomedical educator with Regenerative Medical Supply”
There are so many half and mistruths above that it will take a full blog to debunk it all. So let’s start at the beginning.
Who is Lenora Hughes?
It wasn’t hard to find out who Lenora Hughes is as her Linkedin profile says:
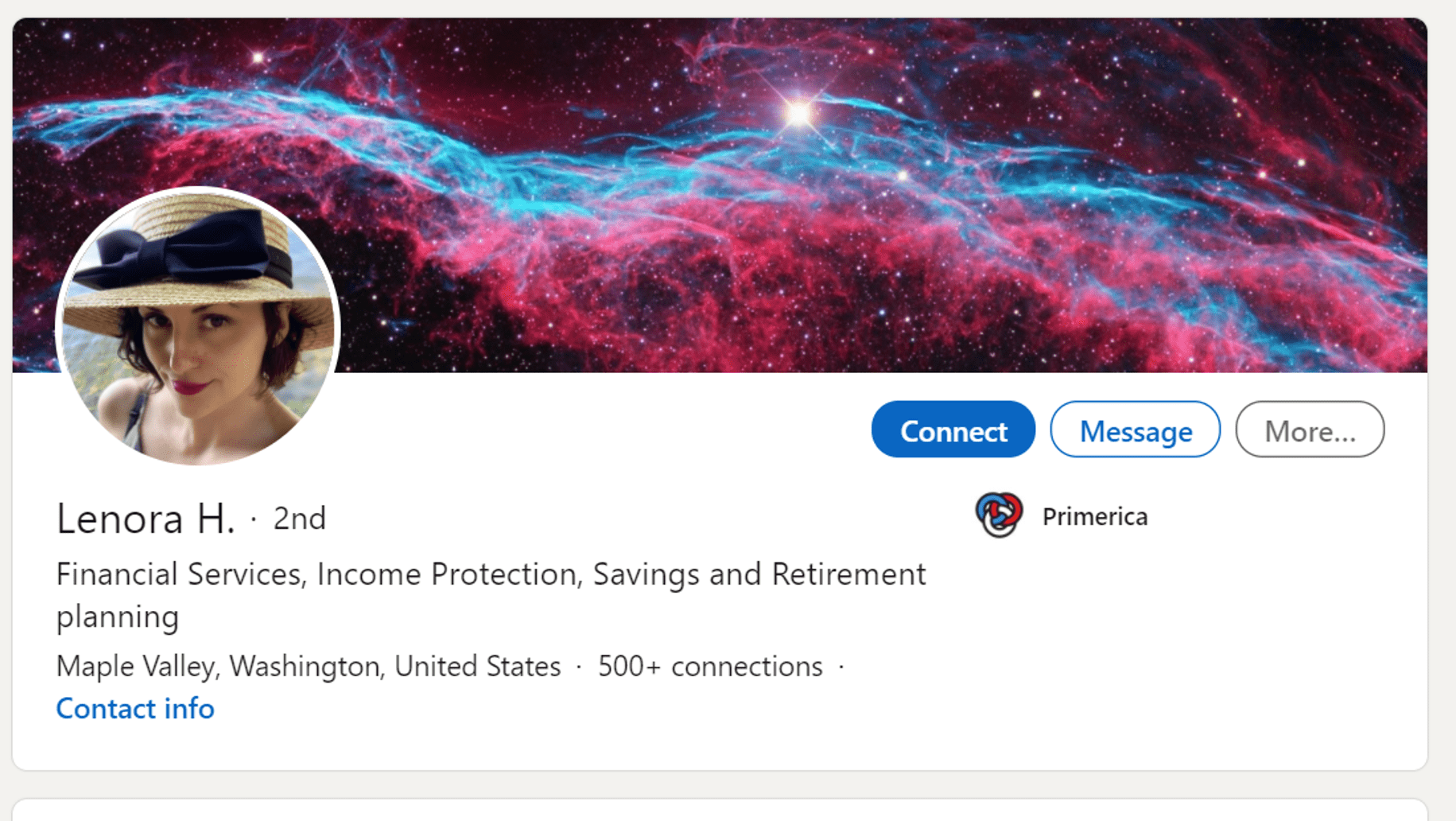
So Lenora Hughes is a financial planner? Maybe this is the wrong one? Nope, in addition to being a financial planner and insurance salesperson for Primercia, she also lists that she is an “Orthobiologic Territory Sales Manager” for the greater Seattle area for “Regenerative Medicine Supply”. Her other past jobs? Selling supplements and being a chiropractic assistant. Her formal education? She lists none on her Linkedin page.
Peeling Back the Misinformation
Lenora’s message to Donna has more misinformation than actual information. So let’s dig into that stuff one by one:
Stem cells are covered by insurance through a different company.
What Lenora is referring to here is what I now call a “Q-code” scam. Basically, a company like amnio technology or another company gets a product reimbursement code for Medicare which is only valid for already covered and grandfathered clinical uses like in wound care or certain surgeries. None of that has anything to do with knee arthritis, shoulder pain, or back pain. However, reps like Lenora then try to convince physicians to bill Medicare or other insurances for these non-approved uses. Medicare will pay pretty much anything if you use the right codes but getting paid to use an amniotic tissue injection to treat knee arthritis is not per Medicare guidelines, so knowingly submitting a false claim is illegal and punishable by 10-years in federal prison per event. To learn more on how this scam works, see my video below:
Regenexx does not have FDA clearance and insurance approval like we do.
This statement has so many different untruths they need to be broken down.
- First, Regenexx is a provider network that uses PRP and Bone Marrow Concentrate, which are both substances that fit squarely within FDA’s 21 CFR 1271.15b “Same Surgical Procedure Exemption”. That means that there is no FDA oversight or approval for these autologous substances.
- Second, Regenexx has actual contracts with hundreds of self-insured companies with millions of covered lives. Those companies contract with us to save money on their orthopedic spend.
- Third, the products that Lenora Hughes is selling have not received any approval, review, or clearance from FDA. They are all registered as a 361 tissue in a 45-minute online registration. The FDA never reviews or clears the product.
- Fourth, as you can see above, there is no legitimate insurance or Medicare coverage for what Lenora is selling.
Our company has invested time and money over the last five years to be the only stem cell product on the market that IS covered by Medicare and most Insurance now. Tricare also covers us, but is covered at 100%.
Again, it’s untrue that any of the products that Lenora sells are covered by Medicare, Tricare, and most insurance plans when used to treat problems like knee arthritis or pain, see above.
It is approved for ALL joints.
Again, as discussed above, amniotic tissues would only have a grandfathered in approval for the treatment of certain non-healing skin wounds and certain eye surgeries like corneal defects. Here are coverage documents for all major health insurers for amniotic tissue treatment of any orthopedic conditions:
- United Heath Care – NOT COVERED for all orthopedic conditions
- Anthem – NOT COVERED for all orthopedic conditions
- Capital Blue Cross – NOT COVERED for all orthopedic conditions
- BCBSMA – NOT COVERED for all orthopedic conditions
- BCBS of Florida – NOT COVERED for all orthopedic conditions
- Wellmark (Iowa and South Dakota BCBS)– NOT COVERED for all orthopedic conditions
- BCBSRI – NOT COVERED for all orthopedic conditions
- BCBS Kansas – NOT COVERED for all orthopedic conditions
- Horizon BCBS – NOT COVERED for all orthopedic conditions
- Federal Employee BCBS – NOT COVERED for all orthopedic conditions
- Blue of California – NOT COVERED for all orthopedic conditions
- HCSC (Illinois, Texas, Montana, New Mexico, and Oklahoma’s BCBS) – NOT COVERED for all orthopedic conditions
- Highmark – NOT COVERED for all orthopedic conditions
- Cigna – NOT COVERED for all orthopedic conditions
- Aetna – NOT COVERED for all orthopedic conditions
- Humana – NOT COVERED for all orthopedic conditions
- BCBS of Minnesota – NOT COVERED for all orthopedic conditions
- Premera – NOT COVERED for all orthopedic conditions
- BCBS of Tennessee – NOT COVERED for all orthopedic conditions
- Capital Blue – NOT COVERED for all orthopedic conditions
Please talk to your Dr about us and share that link below with them. Our product is one your primary care Dr. can use to regenerate your own cartilage with a simple 15 minute injection.
First, if Donna’s primary care doctor were foolish enough to bill Medicare and happens to get paid for injecting her joints, then that doctor is a prime target for a RAC audit to pay back the money. If that doctor knowingly bills Medicare despite understanding that Medicare doesn’t cover this stuff, then federal prison time can be added.
The claim that any of the products being sold are either “stem cells” products (as discussed above) or that they can regenerate cartilage is frankly ridiculous. First, the products that Lenora sells are PalinGen Flow and BioLab Fluid Flow. Based on the extensive research already published and presented, these products like all amniotic products being sold don’t contain live or functional stem cells (1-3). Second, there is no published clinical data that shows that either product, when injected into any joint, will regenerate cartilage.
This is Not the First Time I Have Caught Lenora Poaching Patients
In July of this year, this sales sent a similar message to a different patient who had liked a Regenexx post. Hence, I suspect that Lenora does this quite a bit.
The upshot? If you get pinged on Facebook and sold magic stem cell products from amniotic or umbilical cord tissue with a claim that Medicare, Tricare, and most insurers will cover the cost of your treatment, THIS IS A SCAM. Please be careful out there!
_______________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.