Medicare Umbilical Cord and Amniotic “Stem Cell” Scam Is Crashing
I’ve blogged a number of times on manufacturers who found a creative way to make it appear that their umbilical cord and amniotic “stem cell” products were covered by Medicare to treat orthopedic and spinal problems. This scam is now ending and recent information from CMS provides hard evidence of that change. Let’s dig in.
Fake Umbilical Cord and Amniotic Stem Cells
I’ve blogged on this scam more times than I can count. Bottom line, there are NO products sold in the US today as an umbilical cord or amniotic tissue that contain live and functional mesenchymal stem cells. see my video below to learn more:
The Q-Code Billing Scam
I’ve also blogged quite a bit on this scam. Basically, umbilical cord and amniotic tissue manufacturers figured out that if they changed their Medicare product descriptions for product reimbursement (Q-code) from skin wound care (something that is covered) to add in descriptions of orthopedic indications, CMS wouldn’t notice and would approve the request. The problem? First, the CMS HCPCS Q-code description is meaningless because coverage is actually determined by something called the LCD (guidelines for coverage), and these products aren’t approved by Medicare for orthopedic use. In addition, the second problem is that eventually, CMS was going to catch this scam. See my video below for more information:
CMS is Alerted
CMS runs Medicare and it didn’t take long before they closed this loophole. For example, as early as last year a company called Predictive Biotech applied for a Q-code for orthopedic use and was promptly turned down. In addition, because of these blogs, I was contacted by a Fraud and Abuse contractor for Medicare who was already noticing this issue spiking birth tissue reimbursement claims. They are in the process of ending the billing of these products and seeking reimbursement (called a clawback) from providers.
The CMS Q-Code Rubber Stamp is Now Revoked
This table comes courtesy of our lead research scientist, Neven Steinmetz:
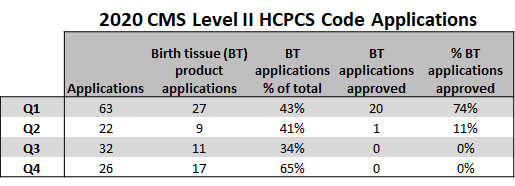
What’s this? This table represents the results of the meeting where birth tissue manufacturers can apply for a Q-code to get their product reimbursed by Medicare. Now again, realize that this should be for the healing of things like diabetic skin ulcers and NOT orthopedic applications. Notice that the percentage of applications approved for anything was 74% in Q1, 11% in Q2, and that by Q3 of last year, nothing was being approved.
Why is this happening? Because the FDA is now taking a bigger role in its veto on this CMS committee. It has clearly stated that these products are unapproved drugs and as such, CMS has no business approving any applications for Q-code product reimbursement.
This is a list of some of the companies that were denied:
How About the Companies that Have Existing Codes?
Again, the companies directing doctors to bill Medicare for birth tissues to treat orthopedic applications are under active investigation by Fraud and Abuse contractors for CMS. That means that any provider billing this stuff and inadvertently getting paid will be subject to clawbacks. That’s a letter coming from CMS that says, pay us X amount (usually hundreds of thousands to millions of dollars) in the next 90 days or we’ll see you in court. By the way, if you lose this case, it’s 10 years in federal prison for every false claim submitted to Medicare. No fun to get one of these letters as a doctor.
The upshot? CMS is closing this scam loophole for Q-code reimbursement. In addition, the companies that successfully scammed the federal government out of likely hundreds of millions of dollars in ill-gotten proceeds are going to have a very bad year. For the providers gullible enough to bill Medicare for this stuff, you can expect you’re nightmare letter soon.
If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.