Marrow Cellution: Playing Games with Bone Marrow Data in New Jersey
Credit: Shutterstock
Since Regenexx has the largest dedicated interventional orthobiologics network that uses highly trained physician subspecialists, there isn’t a week that goes by that someone wants to take a potshot at the big guys. I ignore most of these, but every once in a while, I engage that provider in order to educate my readers. This morning’s example is a physician in New Jersey who is using an expensive trocar called the “Marrow Cellution” claiming that what he’s doing is better than anybody else, including Regenexx. Let’s take a look in-depth at that and why his claims are in my opinion silly.
This blog was originally posted in 2017, but based on feedback this past week from a patient, I edited it and updated the information.
Your Doctor’s Knowledge of Stem Cells Is Largely Learned by Interacting with Sales Reps
It’s hard to explain to someone not involved intimately involved in medicine just how much influence device manufacturers have over physician education. In the area of stem cells, since doctors get very little, if any, education on stem cells in medical school, residency, or fellowship, they only have two ways to get educated. One is to go to courses, but these take time out of the practice. Also, there are few in-depth courses to take, and much of that education is controlled by device manufacturers. Add in that for two years the pandemic put a damper on in-person conferences, and it’s no wonder doctors increasingly rely on the sales reps that come into their office for their education.
What’s the problem with getting educated by sales reps? As you might imagine, somebody trying to sell you something isn’t going to give you the whole story. It’s basically like chucking the consumer reports or reviews on a new car and letting a used car salesman educate you.
This morning I’ll cover a New Jersey physician using a device to draw bone marrow that he claims doesn’t need to be concentrated. I’ve seen some physicians use this device, but as I’ll show you, as the English would say, “It’s a few sandwiches short of a picnic”.
The Silly Claims—Round 1
The New Jersey doctor is named Marc Silberman, M.D. and he claims that he uses a “system” to get more stem cells. He first appears to compare his “system” to other commercially available bedside centrifuges. This table is from his website:
| System |
CFU-f per mL |
| Dr. S’s System |
3290 |
| Harvest BMAC |
1270 |
| Arteriocyte Magellen |
510 |
| Biomet BioCUE |
134 |
| Traditional Trocar |
356 |
Understanding where this data comes from and what he’s talking about here is important. First, this is a copy/paste of a white paper produced by a company that makes a fancy trocar called the “Marrow Cellution” A trocar is the type of needle used by physicians to draw bone marrow. Second, a CFU is a colony-forming unit, which is a rough metric of the number of stem cells in a sample.
The Marrow Cellution trocar is a device that allows the doctor to take small samples of bone marrow as the doctor drives deeper into the bone at a single site. The concept is that it will increase the number of stem cells drawn using this method. Does it work? We’ll examine that issue today.
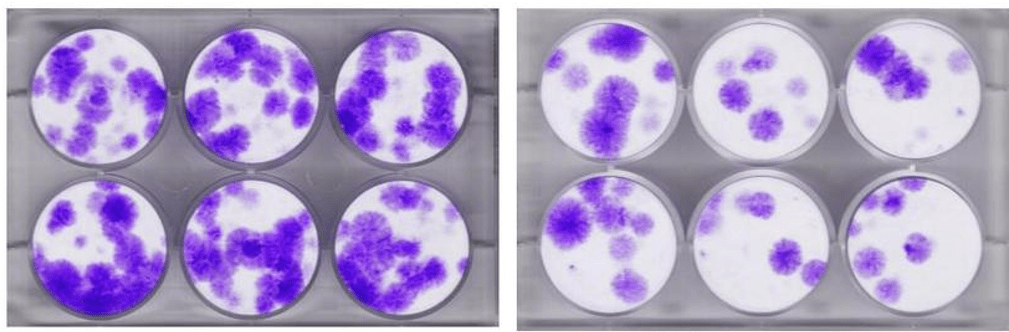
To delve a bit deeper into the table above, a CFU measurement can be taken when you seed bone marrow on a plastic plate and then grow that in an incubator. The stem cells will attach and form colonies. So the more colonies (CFUs) you get the more stem cells that are in the sample. Above you see an example from our research lab. Those purple circular structures are each counted as a CFU-f.
The table claims that the Marrow Cellution device that Dr. Silberman uses yields a lot more stem cells than several different bedside centrifuge systems. To be clear, what’s being compared here is the number of CFU-f’s that Dr. Silberman claims he can pull using only the Marrow Cellutions device versus the number reported by these manufacturers. However, Dr. Silberman doesn’t tell you that this data is really comparing apples and oranges.
First, these numbers weren’t collected by Dr. Silberman who has no internal lab run by Ph.D.s as we do at Regenexx. In fact, they come from a company white paper produced by the manufacturer of the Marrow Cellution device. I know this white paper well, and, regrettably, it’s useless data.
The problem is that the physician author of this advertising piece never compared these different systems head-to-head, but rather copied/pasted the CFU values reported by the companies making these bone-marrow-concentration devices. Why is this an issue? There are dramatic differences in the number of stem cells between subjects based on age, sex, activity, and so on. Also, the number will vary with how many sites you draw from and the volume from each location. Since none of this data is reported or, if reported, it’s not controlled, in this comparison, the table is meaningless. So if company X drew a single bone marrow site at high volume in an older patient and company Y used the same method in a younger patient, we would expect these numbers to be very different. Written another way without medical jargon, the table is like reporting lap times for various race-car drivers to compare their performance. However, the times you report are from different tracks, under different conditions (dry or wet), with the drivers in different make and model cars! Hence, the comparison is meaningless.
In addition, a CFU-f is not a metric that can be compared side to side when it’s from different labs. What you get depends heavily on how many cells you seed into the flasks, how long they’re grown, and how the colonies are counted. I have already blogged on problems comparing CFU-f numbers from different labs.
To do this study correctly, you would need to use one patient and draw both sides in the same way and then process that bone marrow aspirate in different machines. Or in this case, compare the Marrow Cellution trocar on one side and then a standard trocar on the other, using them in the same way. Only then would this comparison mean something. So right off the bat, buyer beware.
The Volume Problem
Another problem with the Marrow Cellution trocar is that it’s only designed to draw 8 ml of marrow. This has changed since the device hit the market, and I suspect the change was made to buff up the numbers. Let me explain.
We’ve known through multiple studies since the 1990s that drawing low volumes of marrow can get you a high stem cell concentration (cells/ml) (1-3). We’ve also known that stem cell concentration reduces as you draw more volume. When the marrow Marrow Cellution device first came on the market, it was advertised as a complement to existing stem-cell-concentration devices. Meaning that you would use this device to perform a typical 60 ml bone marrow aspiration and then further concentrate the cells. However, based on what I understand, the high cost of the device caused physicians to complain. After all, the device cost 20 times more than a standard trocar! Then the marketing changed. All of a sudden, the recommendation was to draw less volume and skip the concentration step, basically allowing doctors to replace the cost of the bone-marrow-concentration kit by buying the Marrow Cellution device.
However, there’s a problem with the Marrow Cellution low-volume approach. While the concentration of cells declines as you draw more from a single site, the overall number of cells increases. So while you can get a high concentration of cells by drawing 8 ml from a number of sites deep in the marrow (what the Cellution manufacturer recommends), any doctor with a simple trocar can likely beat the overall number of cells simply by using it in the same or a similar way (drawing more sites at lower volume). If he or she then has a good machine or protocol to further concentrate those cells, it shouldn’t be hard to get more cells than a Marrow Cellution-device draw.
Our Testing of the Marrow Cellution Device
We were very interested in this device, so we tested it in our clinic and blind tested the samples in our lab. After all, if it really did get more stem cells through some unexplained and understood magic we wanted to know. Hence, we lined up several patients where we used the Marrow Cellution device on one side and a standard trocar on the other. This is what we found:
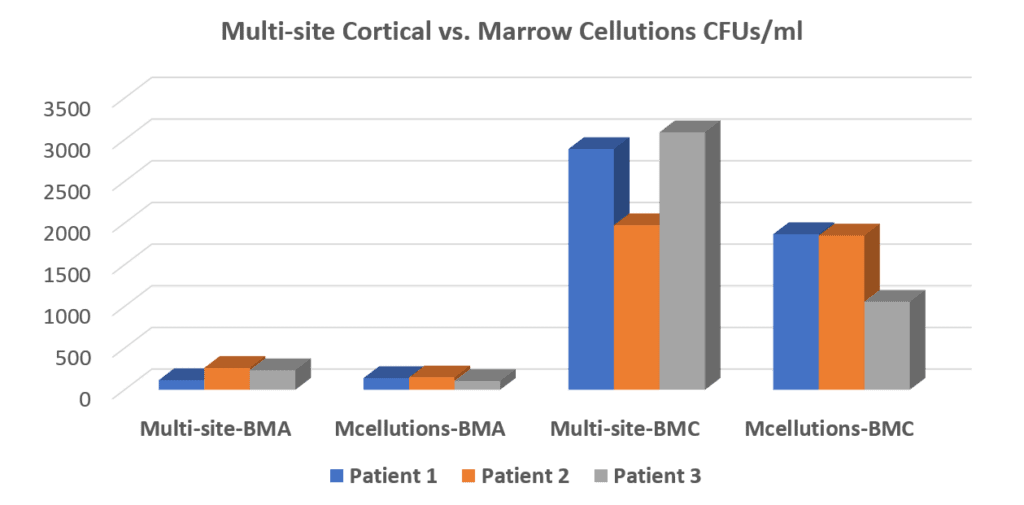
In the graph above of our data, the number of stem cell colonies (CFU-f) found in each of these samples is represented by the height of the bars. To the left, you see the “Multi-site BMA” (our approach) compared to the “Mcellutions-BMA” which is the Marrow Cellutions device. At that point, before concentration, the standard Regenexx bone marrow aspirate procedure produces more stem cells than the Marrow Cellutions device, but the difference is not yet dramatic. Now, realize that this is as good as it gets for the Marrow Cellutions device because there is no additional concentration, meaning this is the cell number injected back into the patient. The third series of bars called “Multi-site-BMC” is the number of stem cells in a Regenexx procedure after we concentrate and isolate two stem cell fractions in the bone marrow. As you can see, that number is many times the amount that the Marrow Cellutions device produces. The bars on the far right labeled “Mcellutions BMC” were just for fun, as we wanted to see what would happen if we tried to concentrate what came out of the Marrow Cellutions device using our usual process. Regrettably, that disappointed.
In the end, based on these poor results for the Marrow cellutions device used by Dr. Silberman, we decided to not go in this direction. Again, rather than taking the word of the sales rep or a poorly done advertising white paper provided by the rep, we decided to test this thing.
The Silly Claims—Round 2
Dr. Silberman, IMHO then goes on to dig the hole deeper. He states the following:
“For example, Regenexx, which can cost $6000, uses pretreatment with prolotherapy
injections the week of the procedure, six to ten puncture sites in your iliac crest, 60 to
150cc of bone marrow aspirate which hurts, and then manual separation with additives
and centrifugation or spinning of the marrow aspirate, plus the need for PRP therapy to
be performed with the procedure.
Dr. Silberman’s method means less cost, less time, less pain, less risk of infection, less
risk of allergic reaction, less waste, better numbers.”
Let’s take this one statement at a time. Regenexx uses a multisite draw. True, we do this because it dramatically increases the number of stem cells as discussed above. As shown above, there is no credible data that the trocar used by Dr. Silberman will increase stem cell numbers and in fact, when we tested it in our lab it produced far fewer cells.
Dr. Silberman also states that our marrow-draw method is painful. While I don’t see any information on his website that describes how much pain his patients feel when he digs into their bone marrow with this doohickey, you can find that information on our website. Way back before Dr. Silberman first ventured into using bone marrow stem cells,(in 2009), we performed a study that found that in 44 consecutive patients, about 9 in 10 thought that the bone-marrow-draw procedure we use was comfortable. I also used the Marrow Cellutions device on three of my patients in the study above. Two of the three complained that it was more painful than the Regenexx BMA side. Why? It’s larger than the device we normally use and goes far deeper into areas I can’t easily numb.
Dr. Silberman points out that we use a proinflammatory preinjection and highly concentrated PRP. Yes, that’s correct. In fact, we’ve published a large chunk of the world’s medical literature on orthopedic stem cell use using that technique. Curiously, I somehow can’t find a single paper using Dr. Silberman’s stem cell technique in the US National Library of Medicine or on his website. Why? Near as I can tell, this procedure has never been studied and published. In addition, you can click here and be taken to live outcomes on our novel procedure by body area, but I can’t find any outcome-based registry data on Dr. Silberman’s website.
Finally, it looks like Dr. Silberman is offering some discounts on his procedure. That’s great, but given that the device he uses produces far fewer stem cells and he has no research at all showing what he does works, IMHO it’s buyer beware! You get what you pay for…
The Silly Claims Round 3
Dr. Silberman claims that the Marrow Cellutions device is “FDA Approved”. In fact, it’s not FDA approved. The device, like all in this space, has a 510K clearance. That means that the company performs no clinical trials, but claims that its device is “substantially similar” to one already on the market. The FDA doesn’t review the claims that the device is better than any other device, merely that it’s similar enough to go on the market. That process takes about 6-12 months on average. That’s quite different than an “FDA Approved” drug that goes through extensive clinical trials costing tens of millions of dollars over 5-10 years and then if FDA decides the drug works, it becomes “FDA Approved”.
Regenexx and the Little Guys
Dr. Silberman’s website highlights the difference between Regenexx and a small practice like his NJ Sports Medicine Clinic. At Regenexx, we test every device independently at our expense in our lab. So we would never allow one of our doctors to advertise that they use this fancy trocar and that this produces more cells until we verified that it actually produces more cells.
The upshot? At the end of the day, we have tested the device Dr. Silberman is using. It doesn’t produce more stem cells. Hence, we’ll stick with the procedure that does produce more cells for our doctors.
This blog was first published on April 23rd, 2017, and then extensively updated on 5/4/22.
_______________________________________________________________________
References:
(1) Batinić D, Marusić M, Pavletić Z, Bogdanić V, Uzarević B, Nemet D, Labar B. Relationship between differing volumes of bone marrow aspirates and their cellular composition. Bone Marrow Transplant. 1990 Aug;6(2):103-7. PMID: 2207448.
(2) Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997 Nov;79(11):1699-709. doi: 10.2106/00004623-199711000-00012. Erratum in: J Bone Joint Surg Am 1998 Feb;80(2):302. PMID: 9384430.
(3) Fennema EM, Renard AJ, Leusink A, van Blitterswijk CA, de Boer J. The effect of bone marrow aspiration strategy on the yield and quality of human mesenchymal stem cells. Acta Orthop. 2009 Oct;80(5):618-21. doi: 10.3109/17453670903278241. PMID: 19916699; PMCID: PMC2823327.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.