More Cord Product Craziness: More CoreCyte and First StemShot Review
As I often say, sometimes you just can’t make this stuff up about the Wild West of stem cells. While we’ve had the amniotic fake stem cell therapies being pushed by companies, sales reps, and clinics, the newest craze is umbilical-cord-tissue treatments claiming live stem cells. These are as much fake “stem cell” therapies as the amniotic treatments and just as illegal. What’s weird is that these companies have real civil and criminal liability by claiming to be stem cell therapies, but they all seem to be fighting to use the moniker because it drives “get rich quick” sales. Case in point this week is a company that has launched an umbilical cord product claiming live stem cells called “StemShot.” This group looks to have exposed a retraction from a university that tested another cord product called CoreCyte. If this retraction is accurate, then our review of problems with CoreCyte’s claims of mesenchymal stem cells that I revealed in February were just confirmed by the University of Utah. Let me explain.
Why the Cord Blood Mesenchymal Stem Cell Claims Are Weak
First, we wouldn’t expect cord blood to have mesenchymal stem cells (MSCs) as only between 10–20% of all umbilical cords yield any MSCs. For more info, see the video below:
The Running Joke on Viability
As you have read here, for those in the know, who understand the science behind stem cells, the viability of amniotic and cord blood stem cell products is a bit of a running joke. These products don’t have viable and functional cells, for lots of good reasons. Despite this, some manufacturers have produced white papers that purport to show that these products do have loads of living mesenchymal stem cells. To get a primer on why viability of these products is a running joke, check out my video below:
Despite third-party tests showing no viable cells, some of the companies that manufacture these products have found out that if they claim that their products have living stem cells, gullible physicians and alternative health providers will believe these claims and purchase more product.
Running Afoul of FDA
Many physicians and chiropractors who use these products claim that they have live cells because someone told them that this was the case. However, despite being dead-cell products and being regulated as such, merely the claim of live cells bumps these products from a 45-minute, free online tissue registration with FDA to a cell drug. In the latter category, each of these products would need a 100 million dollar and 5–10 year FDA approval with real clinical trials per indication (i.e., approval for shoulder, one for the knee, one for ankle, etc.). Since the companies didn’t do this, the claim of live cells makes these products an illegally marketed drug with laws that are being violated that have very real teeth. To learn more, see my video below:
So given that simply claiming that a dead cell product has live cells is illegal and that the manufacturers could be punished for this claim, it’s really bizarre to see certain companies push this envelope. Even more bizarre is that since the physicians and alternative health practitioners buying and promoting them have the same legal liability, these health care providers seem oblivious to their own risks. To learn more about the FDA problems that doctors, chiropractors, and acupuncturists can cause by advertising a dead-tissue product as having live cells, see my video below:
The Most Recent War Between Cord Blood Vendors: CoreCyte
A while back I did a deep dive into a cord blood product’s white paper called CoreCyte. The reader’s digest summary is that the flow cytometry testing had the same problems as all of the white papers I had reviewed on other amniotic and cord products. It was not specific enough to identify MSCs. Despite this, the authors of the white paper claim that the flow data shows millions of viable MSCs per vial. In particular, what seemed odd was that the report seemed to be authored by a university.
First, flow cytometry is a technology that uses LASERs to excite fluorescent dyes on antibodies that bind or don’t bind to certain specific markers on the cell surface. By looking at what’s there or not there, the technology, when interpreted correctly, can identify or rule out a cell type. For MSCs, there has to be a combination of markers present and absent. However, the CoreCyte report only used a fraction of the markers that were supposed to be present and absent on MSCs, but somehow still concluded that there were millions of MSCs. Hence, we sent an e-mail to the scientist at the University of Utah where the report was apparently produced, stating our concerns. We did this because it didn’t seem likely that a university scientist experienced with flow cytometry would make these types of novice errors or draw these erroneous conclusions. That was back in February, and we didn’t think much more about it until this week when something that may have been triggered by our e-mail hit the Web.
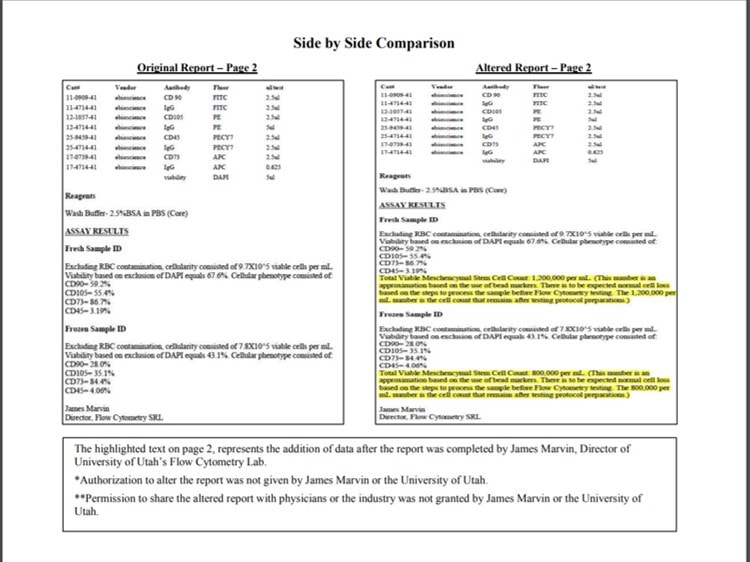
The bombshell report was put out by a clinic called “Go Wellness.” First, I can’t 100% verify the veracity of the PDF listed on their website. I can say that the report fits with what we identified in our CoreCyte review and also fits with our notifying James Marvin from the University of Utah about the bad conclusions of the original report. This is the revised document posted by Go Wellness (click on this to read the document as a PDF):
To review, this new report looks like Dr. Marvin has identified that someone added their own conclusions about his flow cytometry data to make it appear that the original flow cytometry report supported that there were millions of MSCs in the product. Dr. Marvin also states that he never gave permission for the company or anyone else to alter the report. In summary, if this new document is accurate and was issued by Dr. Marvin, it shows that someone altered the original report to support their own conclusions.
More interestingly, as you can see below, I received a message from a sales rep for CoreCyte shortly after this blog went live. He claimed that the retraction from the University of Utah was a fake, created by a disgruntled physician. Hence, I called Dr. Marvin at the University of Utah flow cytometry lab. He confirmed that he did issue a retraction because someone had altered his original report. As he described the report he issued, it matched up with the above retraction. He also stated that at no time did he make any comments or conclusions in his original report about the presence or absence of MSCs.
If this new report is accurate, I have to wonder how many of these university flow cytometry studies have been altered by manufacturers or sales reps. I’ve seen several that look suspicious. Only time will tell there.
Who Is Go Wellness? Acupuncturists Teaching a Stem Cell Course?
What’s more bizarre about these events is that I and other colleagues had commented on LinkedIn about an e-mail blast we all received promoting a new umbilical cord product called “StemShot” manufactured by Utah Cord Bank. First, the name of the product was pushing the proverbial regulatory envelope, as a 361 registered tissue is not permitted to claim that it has MSCs, so using the word “stem” in the product name could be seen as a drug-product claim. Second, Utah Cord Bank seems to be a company that is banking umbilical cords for parents. Third, who is Go Wellness?
Go Wellness is an acupuncture and chiropractic clinic also in Utah. This week, someone put one of their flyers for a stem cell training course on my desk. The course is taught by an acupuncturist who claims to also have an FMP certification. What is an FMP certification? I have no idea and Google doesn’t seem to know either.
So the million-dollar question is, can an acupuncturist inject “stem cells” into a patient in Utah? Not really. This is the Utah code for acupuncturists:
(1) “Administration”, as used in Subsection 58-72-102(4)(b)(ii), means the direct application of an herb, homeopathic, or supplement by ingestion, topical, inhalation, or acupoint injection therapy (AIT), to the body of a patient. Administration does not include: venous injections, immunizations, legend drugs and controlled substances.
So, basically, an acupuncturist can inject an herb, a homeopathic, or a supplement into an acupuncture point. However, as you’ll see, StemShot is a misbranded and adulterated drug product by virtue of its claims of live stem cells. In addition, even if this was an FDA-registered 361 tissue, it would be doubtful that an acupuncturist could inject it based on this language. Finally, even if an acupuncturist could inject it, the law doesn’t contemplate joint injections at all. Yet here’s a stem-cell-injection course being taught by an acupuncturist who also trains acupuncturists? Wait, it gets even weirder.
A StemShot Colony!!!
There is a story about Frank Lloyd Wright that during the depression of the thirties he had little work and that a letter came in from a professor who wanted him to design a house. Ultimately, that letter was seen by the professor on visiting Wright’s studio with a handwritten note scrawled across it by Wright: “Hosanna, a client!” So as you’ll see below, the colony claimed by StemShot seems to fit into that category.
For those of you who follow this blog, there is a metric called a CFU, which means colony forming unit, that defines a rough metric of stem cell quantity. To learn more, see the video below:
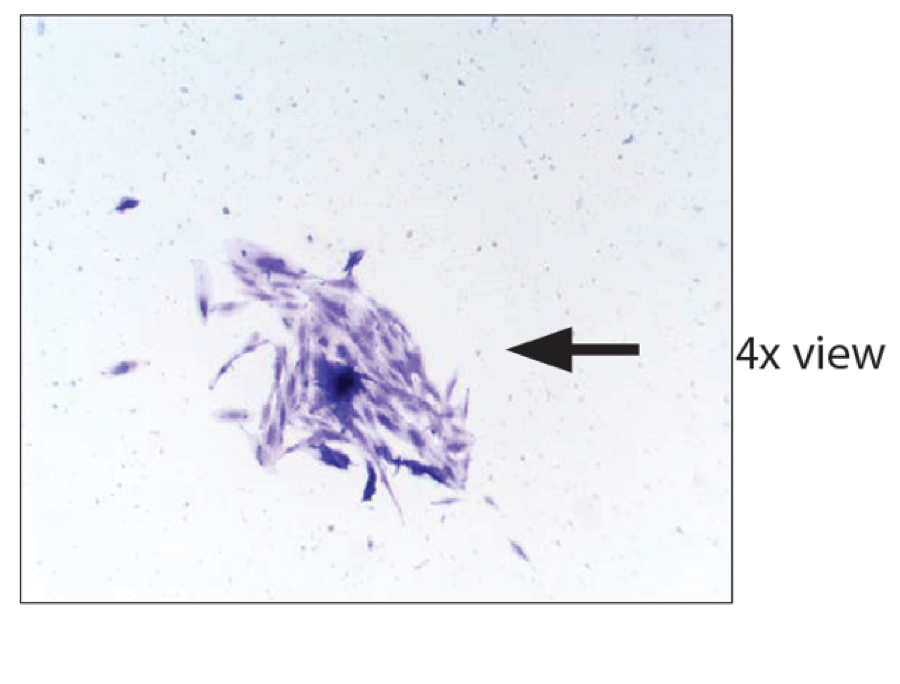
The Go Wellness website recently published this picture as evidence that StemShot had viable cells:
If you look at the colonies in the video above, you can see that they’re circular and contain hundreds of cells each. So is this small clump of tens of cells a legitimate colony? Impossible to tell. We have no additional info, like seeding density, how many colonies were counted (it better be more than just this random clump of cells), what these cells are, how this compares to other cell sources, and so on. The picture at the top of this post is from the Web, showing that the StemShot website is down.
Basically, this information, as presented by Go Wellness, is useless in determining if StemShot has live cells. Even if it has some live cells or even if this small colony is a fluke, just the claim of live cells (i.e., this picture) moves it from a legally marketed 361 tissue into an illegally marketed 351 drug.
The upshot? You can’t make this stuff up! We have a clinic where apparently acupuncturists are contorting their state regulations to practice medicine without a license by teaching how to inject “stem cells.” We now have one umbilical cord manufacturer fighting another over what seems to be altered flow cytometry data, but both, by claiming the presence of live stem cells, are turning their quickie FDA-registered dead-tissue products into illegal cell drugs. Yikes!
__________________________________________
11/1/17—9:36 a.m.: A sales rep has reached out to me to state that he has been told that the above correction was falsified by a disgruntled physician client. I have reached out to the scientist at the University of Utah to confirm the validity of the report.
11/1/17—10:05 a.m.: I just got off the phone with James Marvin from the University of Utah who authored the original report and the correction. The correction above is in fact real. He confirmed that someone had altered his original report to claim that his data demonstrated that live MSCs were in the sample, a conclusion he never reached in his original report.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.