What Is Bioxcellerator?
Bioxcellerator is a stem cell therapy company using mostly umbilical cord mesenchymal stem cells to treat everything from chronic pain to neurological issues, sexual wellness, and rejuvenation. The company has a corporate office in Phoenix, Arizona, but does all of its treatments at their Medellin, Colombia treatment center.
The company aggressively markets its services online in the U.S. In recent months their efforts have relied heavily on C-list celebrity testimonials over significantly quantifiable clinical outcome data.
For example, this is what greets you when you click on one of their ads:
Not All Data On Medical Websites Is Clinical Outcomes Data
Has Bioxcellerator collected and published formal clinical data on treated patients? Well, there’s this:

Is this real clinical outcome data? No, it seems to be more of a marketing survey.
The first clue that this isn’t actual clinical outcome data is the percentages themselves, which are way too high. One of the highest outcome treatments in existence in medicine is antibiotics to treat strep throat, which is only about 85% effective. If you ever see numbers for anything in medicine in the 90%+ effectiveness range, that’s a potential concern. Meaning more context is needed.
So what’s the context?
These numbers represent the percentage of patients that responded in agreement with a statement. We don’t know how many patients this represents, what questions were asked and when, or how much improvement was seen.
Let’s dig in further. The first question is about “energy and vitality.” There isn’t any validated medical questionnaire that measures this very subjective concept. Meaning I’ve got lots of energy and vitality right now as I write this because I downed a healthy serving of green tea an hour ago! For example, after these treatments, how long did this “energy and vitality” last? What validated metric was used to determine this? No answers. Next, we see that 91% of patients who had “diseases, disorders, and injuries” feel better in about a week. How much better? How long did that last? Two weeks? Two months? Two years? We simply don’t know.
Next up, we have 91% of patients reported, “an improved overall feeling of wellness, energy, strength, and cognitive ability.” Again, incredibly vague. My “wellness, energy, strength, and cognitive ability” are doing great right now thanks to my green tea! As above, there is no validated functional questionnaire to measure these snappy concepts.
The last statement with “99% of patients” is VERY DANGEROUS. Being satisfied with the service of the clinic is not the same as measuring if the treatment was effective using scientifically researched and validated outcome tools.
So is ANY of this valuable clinical data? No.
IMHO (in my humble opinion), this is more akin to the type of thing you get from the local car dealership when you take your car in to be serviced and the service manager glad-hands you to make sure you remember to give them a high rating when corporate sends the customer satisfaction survey.
RELATED ARTICLE: Class Action Against Stem Cell Clinic Using Fictional Outcomes
What Real Clinical Outcomes Data Looks Like
For patients, this can be a confusing area. Most generally don’t know the difference between a customer satisfaction survey and actual clinical outcome data collected using validated and standardized questionnaires given at set time points.
One of the things that separate real medical care from unicorns and rainbows is published clinical data. This could be published registry data online or it could be a publication in a peer-reviewed journal. In fact, in looking for a clinic that uses orthobiologics to treat your joint, or spine problems, you need to look for that data.
What should it look like? This is from our treatment registry, which can be easily pulled up on our website:
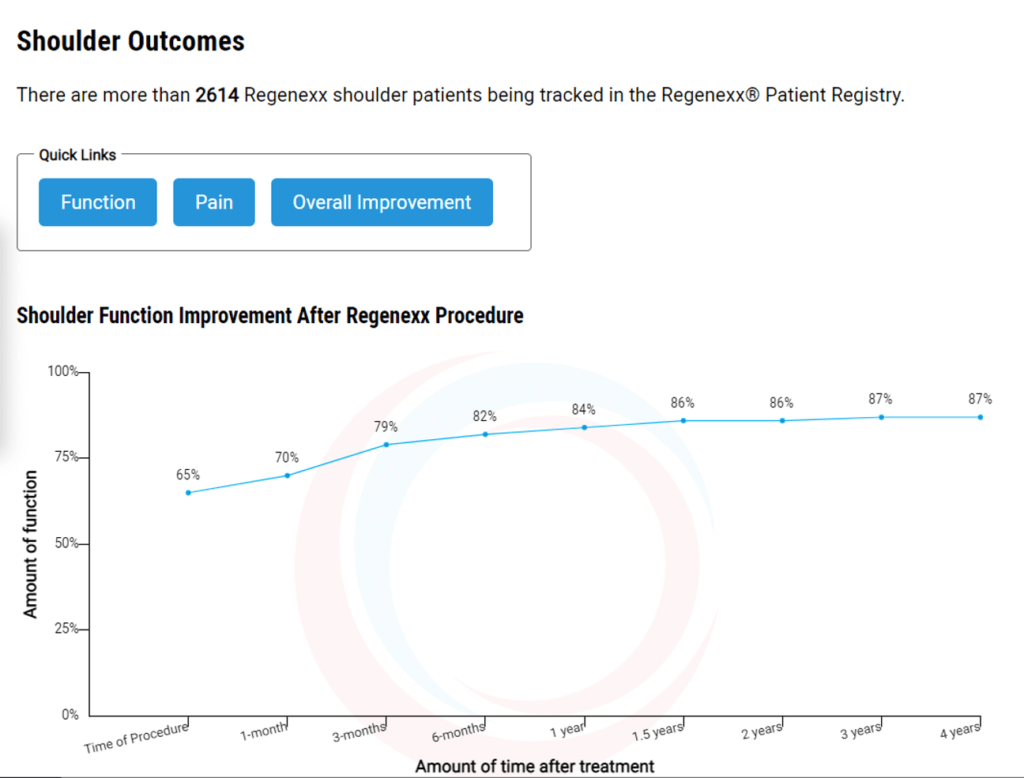
In the example above, you see that shoulder function (here using the validated shoulder questionnaire known as DASH [Disabilities of the Arm, Hand, and Shoulder]) started at about 65% of normal and then got to 87% of normal in 2,614 patients treated for shoulder pain. You also see how this result evolved over 4 years. You can also look at Numeric Pain Score (NPS) or Single Assessment Numeric Evaluation (SANE/Overall Improvement).
What do peer-reviewed publications look like?
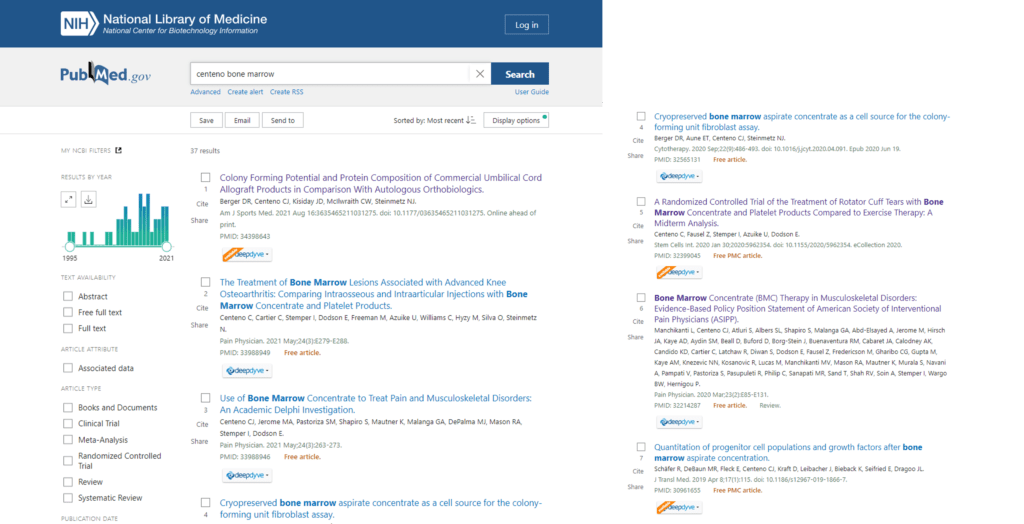
The screenshots above show just a handful of our Regenexx research publications listed in the U.S. National Library of Medicine. Most of this is on patient results. Is there anything like this that I can find on the Bioxcellerator website? No. Anything listed in the US National Library of Medicine under this clinic? No. Anything published by their chief medical officer on mesenchymal stem cells? No.
Diego Correa, PhD
I know Diego, having met him at a medical conference. Brilliant and a great guy. However, this begs the question, what is this very real researcher doing here on the Bioxcellerator website? This is from the clinic website:

Diego studied for his MD/Ph.D. and then post-doc at Yale and then Harvard, but is not associated with those universities. He was an assistant professor at the University of Miami and is now in private industry. I reached out to him because many times when I do that, scientists tell me that they had no idea that they were listed on a clinic website and demand that they’re removed from the site. Diego told me he did some early consulting work for the company and has since demanded that he be removed from this marketing website.
Then we come to this:

I checked online to see if Harvard or Yale had any press release where they reported being associated with Bioxcellerator and nothing came up. Meaning it’s extremely unlikely that this small clinic in Columba has any relationship with these universities. Why are the university logos here? IMHO, likely because a contracted employee studied at these places. It’s my understanding that unauthorized use of these logos for medical advertising is a problem for these universities.
FTC Warning
If the above advertising looks hyperbolic, the FTC agreed by sending a warning letter to BIOxcellerator about its COVID-19 stem cell treatment protocol. The FTC had many of the same concerns I have raised. They stated:
“It is unlawful under the FTC Act, 15 U.S.C. § 41 et seq., to advertise that a service or product can prevent, treat, or cure human disease unless you possess competent and reliable scientific evidence, including, when appropriate, well-controlled human clinical studies, substantiating that the claims are true at the time they are made.”
What Are Golden Cells?

Bioxcellerator has a few websites and one focuses heavily on its product called “Golden Cells.” A term for which there are no publications listed in the U.S. Library of Medicine that states that the clinic or someone else tested or used “Golden Cells” in humans. It seems to be a marketing term made up by the clinic for umbilical cord-derived, mesenchymal stem cells.
Above, you can see that there are many claims for “Golden Cells” like that they promote healing, reduce chronic inflammation, modulate the immune system, enhance sexual wellness, reduce pain, and optimize overall health. The clinic also states that they can treat rheumatoid arthritis, type 1 diabetes, Alzheimer’s, Parkinson’s, Traumatic Brain Injury, spinal cord injury, chronic pain conditions, and CTE. Those are some HUGE claims! Is there any clinical data published by the company that supports these claims? No. Has Dr. Correa published anything that would support these claims? Not exactly. His publications on this cell source seem limited to their use in COVID-19 patients. Was this COVID-19 trial conducted with Golden Cells in Columbia? No. It was conducted at the University of Miami, where he works.
Do we even have basic long-term safety data on using this cell type in humans? Not really. I did find a study that looked at a few hundred cases of patients receiving umbilical cord mesenchymal stem cells for mostly severe autoimmune diagnoses over 4 years (1). These patients had loads of issues after IV infusion including a reported 29.5% infection rate, but the authors reported that they believed this wasn’t due to the cells. Given that these were really sick patients with about 1 in 10 dying during the study period, it’s hard to determine if the cells or their diseases caused these deaths and complications. As for the rest of the literature, suffice it to say that we really don’t have great safety studies in humans on the long-term complications of being treated with this cell type. This is doubly true for the specific sub-type of umbilical cord mesenchymal stem cells the clinic claims it uses.
Physicians
The Bioxcellerator website lists many local Colombian physicians. I looked these physicians up separately, and while many look like credible doctors, almost none even list working for Bioxcellerator. In fact, based on published Linkedin profiles, only a Dr. Ramirez Restrepo lists that he works at this clinic. For example, there are two neurosurgeons and two orthopedic surgeons listed, but from looking at their Linkedin and GMB pages, none of these physicians list that they actually practice at Bioxcellerator. What does this mean? These physicians are likely contractors, paid by the case, and not in the clinic full time or even part-time.
In Summary
I told you this review would be complicated. The marketing claims are over the top and so unsupported by any real clinical data. After reaching out to Dr. Correa, he has asked the company to remove him from their marketing materials.
The upshot? I hope that this clinic actually starts to publish some real data. While umbilical cord mesenchymal stem cells hold real clinical promise, making wild claims about these cells being a panacea to treat everything is why the clinical field of regenerative medicine is often attacked.
_________________________________
References:
(1) Liang J, Zhang H, Kong W, et al. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9(1):312. Published 2018 Nov 14. doi:10.1186/s13287-018-1053-4 [PubMed]

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.