Signature Biologics: Beyond Stem Cells?
Credit: Shutterstock
We’re entering the 5th month after the end of the FDA discretionary period and the vast majority of Amniotic and Umbilical Cord scams seemed to have disappeared. However, as I’ve been writing about periodically, there are still some that persist. Those have tweaked their messaging, but they all really have the same problem. They all need to keep the “stem cell” moniker because that drives sales, but they also know they can’t use it without running afoul of the FDA and/or FTC. This morning we’ll go over the claims made by a sales rep for Signature Biologics, which IMHO are very problematic. Let’s dig in.
Stem Amniotic and Umbilical Cord Cell Scams Pre-May 31st 2021
The FDA set a date of May 31st, 2021 as its drop-dead date for birth tissue vendors (and others) to either file an IND (an application for a clinical trial for a new drug to be FDA approved) or end business operations. Peter Marks, head of FDA CBER very clearly stated that a product couldn’t be sold while the company was in the IND process, so most companies sold their inventories to reps. However, some have persisted in the obvious dodge that because they filed for a clinical trial authorization (IND) with FDA, they could continue to sell their birth tissue products.
What Is Signature Biologics?
I’ve blogged on Signature before. They’re a company owned all or in part by Neil Riordan who is a Physician Assistant and a Ph.D. who runs a clinic in Panama. They have made claims before that they were selling a stem cell product in the US. Their first attempt at a birth tissue product resulted in a letter from the FDA and then a court battle. Their second attempt at that was caught by this blog.
Signature’s New Sales Slogan
Likely in response to the FDA crackdown, Signature Biologics has a new sales slogan, “Beyond Stem Cells”. Let’s dig in below to determine whether that slogan means what it says or is just more of the same.
Are These Umbilical Cord Stem Cell Products Real?
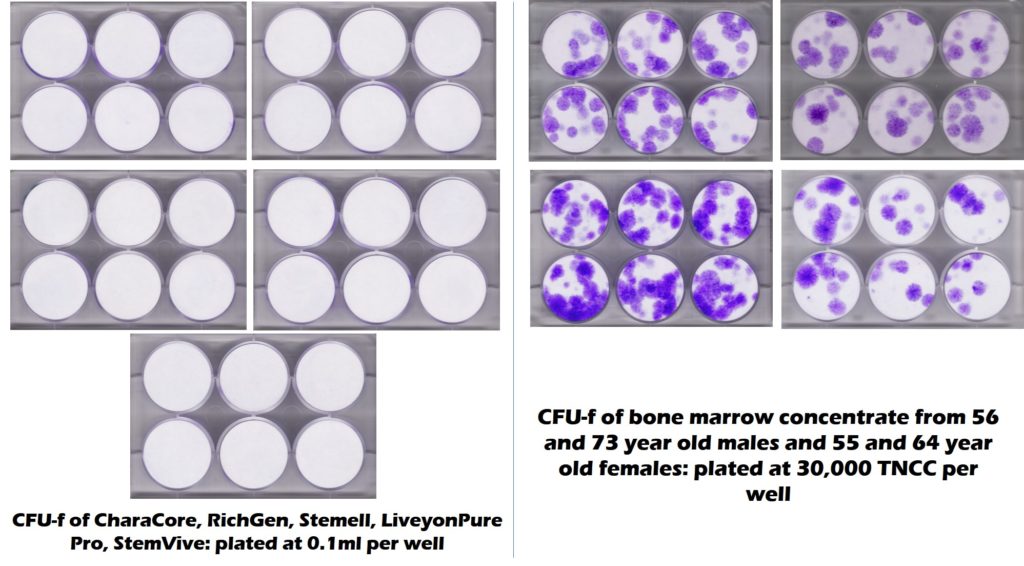
NO. Our lab, working with the CSU Translational Medicine Institute, published a paper in the American Journal of Sports Medicine that tested a number of these products claiming to sell mesenchymal stem cells derived from umbilical cords (4). We confirmed that there were zero living and functional MSCs in any of these products. The money shot from that study is above, showing that while elderly bone marrow contained copious amounts of MSCs (purple dots in this CFU-f test), all 5 products claiming millions of live and functional MSCs had none (all white). Our lab earlier and then other labs have also confirmed these findings after testing Umbilical Cord and Amniotic products (1-3).
The Recent “Go Wellness” and East-West Health Video
I get sent stuff all the time. I was recently sent a video that is from East-West health, a clinic that I have blogged on before because they stole my slides regarding results from bone marrow disc procedures and used them for their own marketing despite not being able, as an acupuncture clinic, to inject the disc. In that blog, I had noted that they made some outlandish claims about the diseases they could treat using birth tissue “stem cells” and sure enough, the FDA graced East-West Health with a nastygram about those claims.
The video begins with Cade Archibald as the interviewer who lists himself as a founder of East-West Health Solutions. He has a four-year degree in finance and his past jobs have included two in construction, and a sales manager. His brother is Regan Archibald, an acupuncturist who co-founded the clinic in Salt Lake City Utah.
Next, we meet Kim Staples, a sales rep for Signature Biologics. She’s worked for Predictive Biotech as well as Applied Biologics, both companies that have made this blog for claims about their “stem cell” products. Kim has a 4-year degree in Hospitality and has also worked in 401K plan sales.
The first thing Kim indicates, in direct contradiction to what Peter Marks said in his AAOM webinar, is that since Signature Biologics has filed for a 351 IND, they can sell their birth tissue product that has a 361 tissue registration without FDA approval. She also interestingly claims that they will have their FDA approval for their knee OA indication next year. That timeline seems like quite a stretch as the usual time period from where they are in the process is at least several years, so likely sometime in 2024 or 2025 is more reasonable.
A cell therapy trials expert searched for this knee OA trial, which the sales rep states is with the Mayo clinic. There were no hits for 2019, 2020, or 2021. This expert couldn’t identify any trial that was active and linked to Signature or the other companies associated with it, let alone one that would be completed and approved by FDA by 2022.
Then she says all of this:
“In the past, we called them “stem cells” because that really just became a buzzword. Yes, there are different types of stem cells, mesenchymal stem cells, native in these tissues and that’s OK, the FDA recognizes that and that’s fine as long as we’re not using these tissues and just isolating the mesenchymal stem cell or culture expanding them. But if we’re leaving them in their native state, minimally manipulating them and using them as cushioning tissue, that’s the homologous use, then this is all compliant with how the FDA regulates these 361 tissue products.
So you’ll hear us referring to and you’ll see in our literature, and I would suggest too that probably in your literature in your marketing you adopt this as well to some extent, because it helps in terms of compliance and it also helps in terms of expectations, of the patients, when they understand how these products work. So we’re using these tissues, these biologic tissues, to transplant as a replacement or a supplement. There’s no complicated pharmaceuticals or invasive surgery, rather we’re arresting the progression of the disease with these homologous tissue supplements.”
So let’s unpack the regulatory problems that are created by what Kim stated. To understand all of this you need to know that the FDA is a claims-made system. Meaning, what Kim claims drives the regulatory pathway for what she’s selling:
- Kim claims that the Signature Cord product has live and functional MSCs. IMHO, that makes this product an illegal unapproved drug product. Meaning, the FDA has made clear time and time again, that a live and functional MSC claim made for a birth tissue product makes it a drug. In other words, that product can’t be sold using the simple 361 FDA Tissue registration that Signature has for its product. Again, the fact that Signature has an IND study ongoing has no bearing on that fact.
- Kim claims that the product she’s selling is “arresting the progression of the disease” which again, is a clinical efficacy claim that makes this a 351 drug being sold without a 351 drug approval.
The New/Old Dodge
Kim uses several sales tactics here that I have seen and blogged on before:
- Convince the buyer that the product has MSCs (even when research shows that these products don’t contain live and functional MSCs).
- Get the buyer to believe that since the company has filed an IND on another product, that it can legally market and sell its other products that only have a 361 registration without any FDA approval.
- Makes claims that are not Kosher with the FDA for a 361 product, but then couch those non-compliant claims in language that is consistent with the FDA 361 regulations (in this case claim that using the world “cushioning” in her product description wipes away the sins of the first claim).
The upshot? Signature Biologics, based on this video and IMHO are still violating FDA regulations with the sale of its 361 Umbilical Cord products. East-West health is still up to the same game it was playing before to got its FDA letter, now just using a third-party sales rep to deliver the same “stem cell” marketing message. So will the FDA crackdown again here? Only time will tell, but IMHO videos like this one clearly create significant regulatory risk for both Signature Biologics and East-West health.
_________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Panero AJ, Hirahara AM, Andersen WJ, Rothenberg J, Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. Am J Sports Med. 2019 Apr;47(5):1230-1235. doi: 10.1177/0363546519829034. Epub 2019 Mar 7. PMID: 30844295.
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Aug 16:3635465211031275. doi: 10.1177/03635465211031275. Epub ahead of print. PMID: 34398643.
If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.