Do Two FDA Warning Letters for Predictive Biotech Represent a Change in FDA Policy?

Predictive Biotech has made it onto the blog a few times, mostly for continuing to insist that it’s Wharton’s Jelly product is stem cell-rich. Hence, while other umbilical cord companies have received a steady barrage of letters from the FDA, I was wondering when Predictive would get their FDA communication. That just happened this week, but they didn’t get just one, but TWO letters. What’s different about the second letter is something that should have all birth tissue vendor companies who are violating FDA regulations and the clinics that use these products very concerned.
Wharton’s Jelly and Stem Cell Claims
Wharton’s jelly is the stuff that gives an umbilical cord it’s flexible rigidity like a garden hose that’s filled with water. When it’s taken from the baby, it has stem cells in it, but by the time it’s processed and bottled and sold it has no viable stem cells. To learn more, see my video below:
Hence, any company that is claiming that it’s selling a Wharton’s Jelly product that claims it contains live mesenchymal stem cells is more likely than not, fraudulently making that claim.
The FDA on this Issue
The FDA has come out very CLEARLY on this issue that any company claiming to be selling stem cells from umbilical cords is not legally selling that product. See the FDA Consumer Alert below:
However, the companies claiming to sell umbilical cord “stem cells” have a very serious problem. On the one hand, most should know by now that the data that has been published and presented at conferences doesn’t support that there are any living and functional stem cells in what they sell. This includes those vendors selling cord blood and Wharton’s jelly. However, their entire business model is built on the idea that they’re selling physicians “stem cells”. That’s baked into their product pricing and their sales strategy. Hence, giving this up voluntarily would mean financial ruin. So, in my opinion, many like Predictive won’t give up the “stem cell” moniker until they are forced to by the feds.
Predictive Biotech
One of the more interesting stories in the saga of umbilical cord vendors telling doctors that they’re selling vials of stem cells is a company called Predictive Biotech. Despite the data out there that doesn’t support that Predictive’s Wharton’s Jelly product called Corecyte has any viable and functional mesenchymal stem cells, it has defiantly kept making claims on its website that it contains mesenchymal stem cells. For example, the same “Product Grid” that has been there for the past several years claiming that CoreCyte has viable MSCs is still up on their website as of this writing. Now there’s also a new product page for CoreCyte that threads a similar needle:
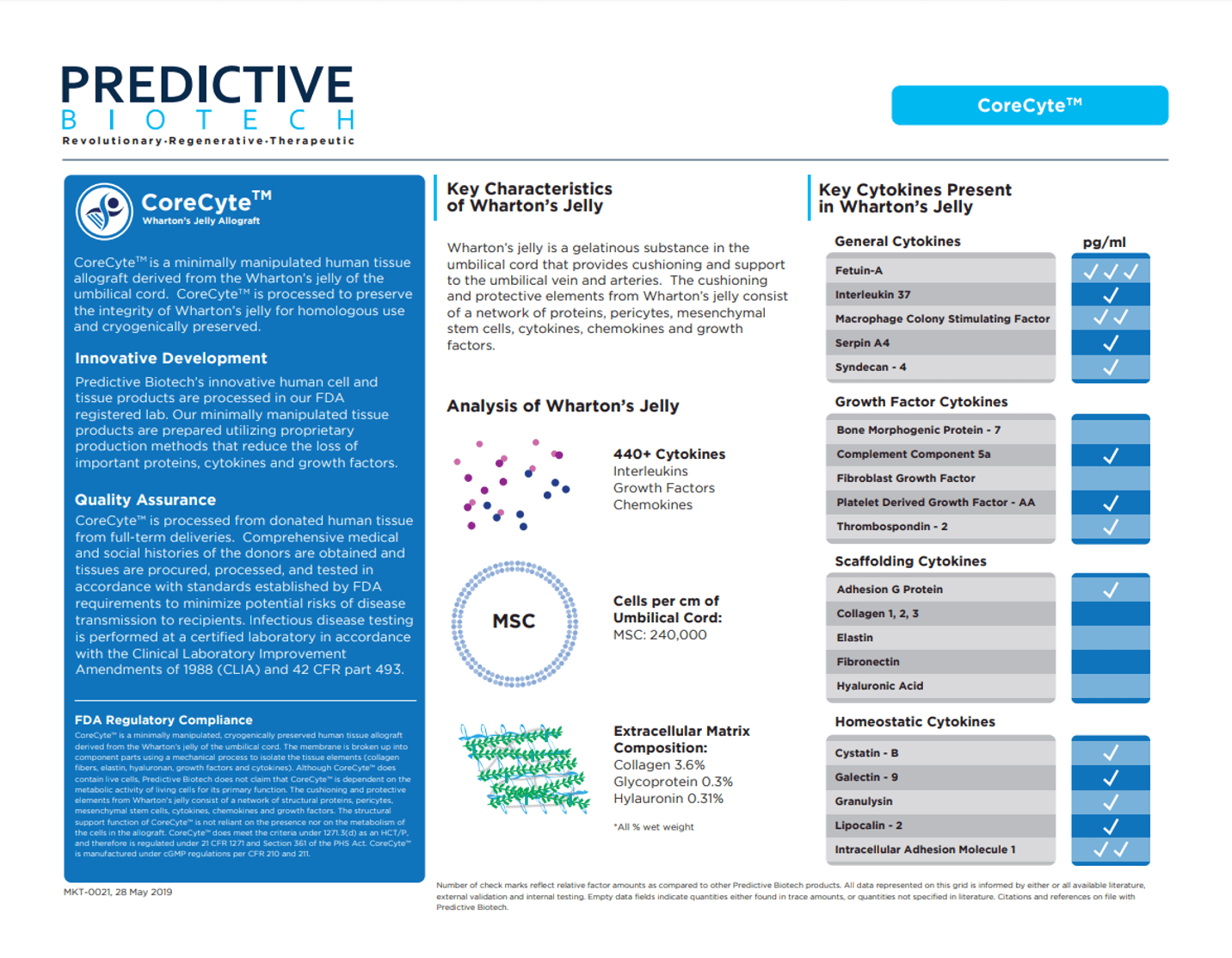
Notice how on this product sheet (click to see a bigger image), the company states that an analysis of Wharton’s Jelly shows 240,00 MSCs (Mesenchymal Stem Cells) per cm of umbilical cord. Now what we don’t see is any claim that this is what’s in Corecyte, hence the “threading the needle” part.
FDA Sends a Warning Shot Across the Bow
The FDA this past week sent two Warning Letters to Predictive Biotech. This was expected, given what in my opinion has been the company’s longstanding non-compliance with regulations. The first was the typical Warning Letter sent to countless similar companies that are claiming to be selling umbilical cord stem cells. The second as you’ll see below was decidedly different and likely represents a new area of attack that the FDA will use in the coming months.
This is the first letter (click on the pic below to be taken to the actual letter):
This is not a pretty letter and you can bet that it will be followed up by multiple FDA on-site inspections of Predictive Biotech’s Utah offices. While what seems to have triggered FDA here are claims that CoreCyte could treat COVID-19, the letter clearly lays out that Predictive’s claims about the product (whether true or not) make it an unapproved drug product. Again, if you insert “Chronic Pain”, “Knee Osteoarthritis”, or “Antiaging” for COVID-19, it’s pretty much like every Warning Letter sent to many different birth tissue vendors violating federal law. However, what was really unexpected and surprising is what was found in the second FDA Warning letter to Predictive Biotech. Before we look at that letter, you need to learn a bit more about what these vendors are permitted to say about their products.
Birth Tissue Vendors Crossing the Treatment Line
One of the interesting and increasingly disturbing recent trends that I have noticed in the birth tissue vendor world is crossing the line into treatment protocols and clinic partnerships. Why is this a big deal? Because these companies, like Predictive Biotech, only have simple FDA Tissue Establishment Registrations. That means that none of them have any FDA approval or review of anything, so what they can say about what their products is EXTREMELY LIMITED. For example, they can say that they offer a Wharton’s Jelly product that could be used by a doctor for cushioning a body part. That’s about it. However, increasingly, to goose sales numbers, these companies have been venturing into treatment claims and protocols. Let’s explore why that’s a HUGE issue.
Once a company with a registered tissue makes a claim about which medical problems that tissue can help, that claim invalidates their simple registration and requires them to get full FDA approval with clinical trials. Hence, they go from a free online registration that costs them nothing to a process that costs hundreds of millions of dollars and takes 5-10 years to get a product to market. Hence, making a treatment claim is the “kiss of death” for these companies.
So how has that been happening? For some companies, it’s posting a video testimonial of patient results on their websites. Others have put out treatment protocols. Still, others have partnered with or own clinics. It’s this last category that brings us to the second Predictive FDA letter.
Why The Second FDA Letter to Predictive is a HUGE Surprise
It seems like the FDA in its game of Whack-a-Mole with birth tissue vendors is always a step behind, but the second letter to Predictive Biotech sent by both FDA and FTC, in my opinion, shows they are now a step ahead. That letter was addressed to a clinic in Pennsylvania who is a “Predictive Biotech Certified Facility”:
My guess here is that the FDA first got wind of this clinic called “PA Green Wellness” making claims that it could treat or prevent COVID-19 with CoreCyte. These clinics in PA and Deleware are actually Medical Marijuana clinics that seem to have added “stem cell therapy”. The “medical director” of the venture is an 87-year-old family physician named Richard Macgill, M.D. Given a review of press releases, he is not the primary owner of these clinics.
The agency quickly linked this clinic back to Predictive. How these two operate together is unknown at this time. Did Predictive take a stake in the clinic? Is it involved in marketing? Does the clinic have an exclusive product relationship in exchange for financial support? I’m not sure it makes a difference in that both FDA and FTC clearly have made a connection and copied the CEO of Predictive on the Warning Letter to the clinic.
The one paragraph in the letter that sums it all up is this:
“Virtually anyone can benefit from a mesenchymal stem cell treatment, however injections are most beneficial if you’re noticing a reduction in your body’s natural healing power . . . or if you’ve experienced an injury. Or suffer from chronic inflammation . . . that needs assistance to heal . . . our products are safe and effective.” [from your website, www.pagreenwellness.com/regenerative-medicine/]”
In my opinion, this statement sums up the whole industry that Predictive Biotech and other companies are now going after. The idea that their products contain “stem cells” and that these products should be injected into healthy people for general wellness. While most credible physicians who understand orthobiologics have gotten the memo that these are not stem cell products, many in that integrative medicine and anti-aging space have not. Hence, they are ripe marketing targets for the stem cell sales pitch.
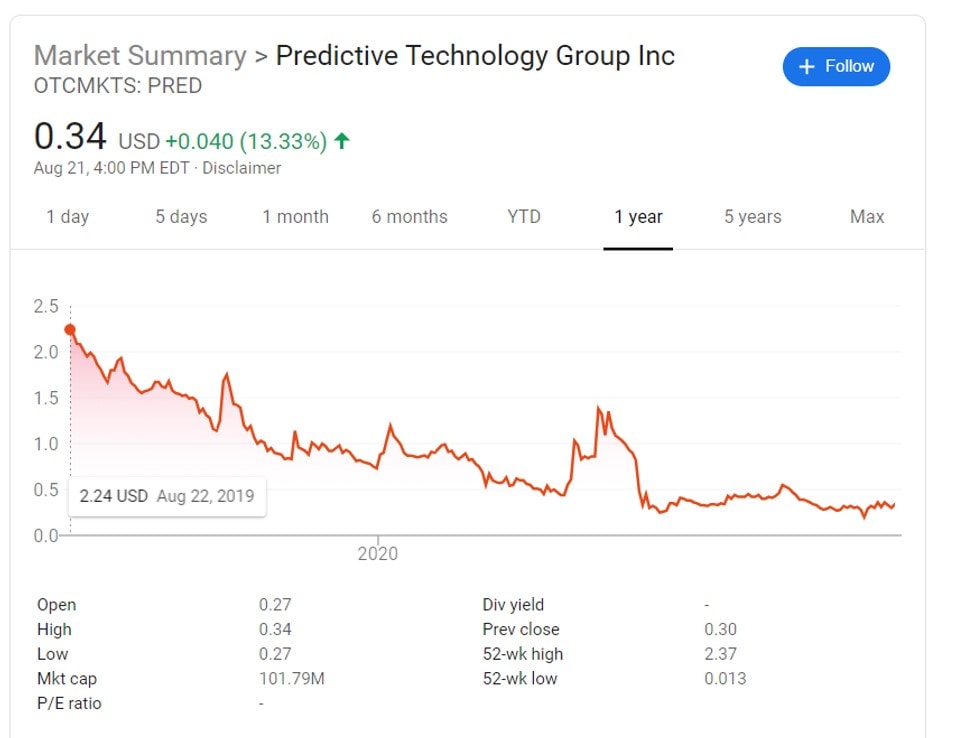
Predictive’s Stock Price
Predictive Biotech ( Stock Ticker: PRED ) is a publically traded penny stock. Early last year it was riding high after aggressive stock sales promotions (see below). Predictive was then featured by a short-seller aptly named “Hindenburg Research” This detailed article focused on the past SEC problems experienced by company executives and what the Hindenburg thought was evidence of inside dealing of stock. I would highly encourage you to read the piece as PRED’s stock price has been on a slow decline ever since that time. Then came a suspension of trading in April due to the company marketing COVID-19 tests that were not approved by the FDA. The stock is now trading for only 15% of its former value.
The upshot? There has been a significant change in FDA’s tack here going after birth tissue vendors by now including the clinics that they partner with on Warning Letters. Here, Predictive seems to have entered the COVID-19 treatment world with a clinic that formerly sold exams for medical marijuana cards run by an octagenarian physician. This change in FDA policy will be interesting to watch unfold, but I would expect more and more clinics to get pulled in with the “bad actor” birth tissue vendors.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.