Profiting from Amnio, Exosomes, and Umbilical Cord Products Right Up to the Wire and Beyond
With the FDA enforcement discretion period rapidly coming to a close, how are some of the bad actors responding? After investigating this, it’s a mix of retreating, garbled messages, and business as usual. In addition, like a game of telephone, the sales reps are making up all sorts of crazy stuff. Let’s dig in.
The Rumors
Several sales reps have contacted me this week either in a state of panic or very happy to see that the companies they knew were non-compliant are having to abandon major product lines overnight. The reps that were in a panic are responding to news from Apex Biologix.
Retreating
I’ve covered a company called Apex Biologix before, which is a sales distributorship. Basically, IMHO, for years they have been profiting from selling non-compliant allograft tissues long after the FDA made it clear that selling these tissues was not legal. This included amniotic fluid, umbilical cord tissue, and exosomes. What is their position today? This is from an email the company sent on May 25th to medical providers:
“As you may already know, the FDA has recently confirmed the ending of compliance and enforcement discretion policy for certain human cell, tissue, and cellular tissue-based products (HCT/P’s). As of May 31st many allograft businesses will be closing or stopping the sales of their amnio products.”
Huh? So effective next week, Apex goes on to say that they will only be selling autologous products. On the one hand that’s great that Apex is finally coming into compliance. On the other hand, Apex sucked every possible dollar out of this cash cow it could and then dumped it all like a hot potato before the FDA hammer fell.
Mixed Messages
Here’s a new one. A colleague sent me this e-mail from sales rep Kevin Spinoza from Regenerative Aesthetics Enterprises who is selling Prime Pro and Prime MSK. Here’s the flyer sent for Prime Pro:
This is very clearly non-compliant advertising by stating that this is a stem cell product. Then this from the email:
“As you may have already heard the FDA is making a significant shift, and enforcing new regulations starting June 1st with regards to regenerative medicine. The FDA is completely removing the 361 category and requiring that any company that is distributing a 351 biologic will require to have both an ongoing IRB study and an IND number assigned to the product.”
I texted and then called Kevin. He and his partner Doug Rabold claimed on the phone that based on what they had been told by their suppliers that the FDA would be creating a brand new 361 category that would allow a company to have an IND filed and sell a product at the same time. I checked this with a regulatory attorney this afternoon and his texted response was “LOL”.
This is from Peter Marks, head of FDA CBER during a recent Q and A online:
“Marks: If a company has an IND can they continue to market the product? No, they can’t. They can continue under IND with cost recovery, but they can’t be, after this point, after May 31st, you either have a license, in which case you can have…that’s legal to introduce into interstate commerce and charge for the product or you have an IND in place in which case you can distribute within interstate commerce, but you can’t charge or your can charge only cost recovery for what it costs you to make that product.”
So we will see what this is all about next week, but I suspect that Kevin and Doug will NOT be selling their amniotic and other birth tissue products for orthopedic use going forward. That is of course until these companies go through a multi-year process of clinical trials, FDA review, and 351 drug approval.
What’s up with the exosome company Kimera?
After years of messaging that the company would was 100% compliant because it was culturing cells to get the exosomes instead of selling stem cells, Kimera has made some changes. They now sell research use only exosomes and a vet product. However, they are still promoting wound healing products. I’ve seen this from Kimnera before and then either the CEO or medical director Doug Spiel would give a recorded lecture about exosomes curing everything under the sun. For example, the site lists that they will be exhibiting at an autoimmunity conference and a stem cell conference. Neither has much to do with wound healing. So will it be business as usual? Will they stick to only wound healing applications? Only time will tell.
Q-Codes Being Rescinded?
In speaking to sales reps this week there is word on the street that the Q-codes with orthopedic indications that were issued in error for birth tissue products are beginning to get canceled by CMS. This is great news if true, as Peter Marks last week stated that FDA was working with CMS to get rid of these codes. This means that Medicare is admitting it issued these codes in error, which is the beginning of a RAC clawback bonanza that will bankrupt many clinics. As I said before, this could be a HUGE 500M to 1 billion dollar claim against hundreds of US medical providers who billed Medicare for these products.
Business as Usual
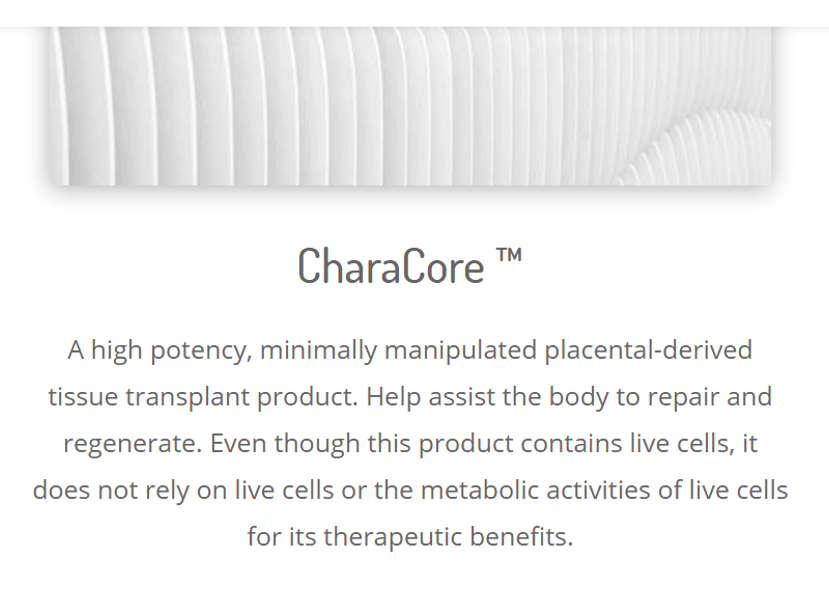
CharaCore
I’ve covered Chara Biologics several times on the blog. This is a reseller that is run by psychiatrist Joy Kong. I went to their website to see if they were compliant now, but this is what I found:
I’ve seen this dodge many times. basically, the sales reps sell the product telling providers that it contains “stem cells” and then the website states that while live cells are present, the product doesn’t rely on them for its therapeutic benefits. The company is trying to thread the needle based on the 1271 regulations which make a similar statement, but I suspect that this careful wording won’t convince many at FDA. Does the CharaCore product have live and functional mesenchymal stem cells? Not according to a paper we just had accepted by the American Journal of Sports Medicine which will be out soon.
Regenative Labs
I would be remiss is I didn’t mention Regenative Labs as they have remained defiant that their umbilical cord product can still be sold and billed to Medicare and that the beginning of FDA enforcement will have no impact on them. Have they made changes? Not as of this week. They continue to sell ProText as a product containing mesenchymal stem cells and that can be used for a cartilage indication. How long will it be before their Q-code gets canceled by FDA? Not long I hope.
The upshot? Next week should be interesting for these birth tissue stem cell vendors. I suspect that while all of this will take a few months to unfold, my prediction is that by fall, there are no birth tissues available for orthopedic use. In addition, with the Medicare RACs chasing after one of the largest Medicare fraud recoveries in US history, a good chunk of the chiro-owned birth tissue clinics and a handful of shady physician-run clinics will be out of business.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.