CharaCore Is Still Making Claims It Can’t Prove…
I’ve blogged several times on a company selling an umbilical cord product that it claims has many millions of stem cells. I’ve shown how independent lab tests have documented that this isn’t true and how a lab report being used to stoke sales was somehow suspiciously altered. Now that company is doubling down in the context of ramped up FDA enforcement on providers using similar products. So let’s dig into the fake world of umbilical cord “stem cell” products!
Umbilical Cord Stem Cell Scams
If you’re a new reader of this blog due to our Coronavirus coverage, then you may remember past blogs that have discussed how unscrupulous manufacturers and physicians have been offering IV umbilical cord “stem cell” treatments to treat or prevent COVID-19. This was all based on a small paper out of China that seemed to show that specially cultured and genetically modified stem cells could help patients in the ICU. The problem? U.S. manufacturers of umbilical cord tissue products, which contain no live and functional mesenchymal stem cells have claimed that their products can be used the same way. Enter a company called Chara Biologics…
CharaCore and Lab testing
CharaCore is a product manufactured by a company run by a psychiatrist is California by the name of Joy Kong. Chara means “Joy”, so the company is called Chara Biologics. I’ve blogged many times on this company because it has consistently claimed to be selling a mesenchymal stem cell product derived from umbilical cords, despite evidence to the contrary.
First, our lab and the CSU Translational Medicine Institute both tested CharCore and other umbilical cord “stem cell” samples and the results are below:
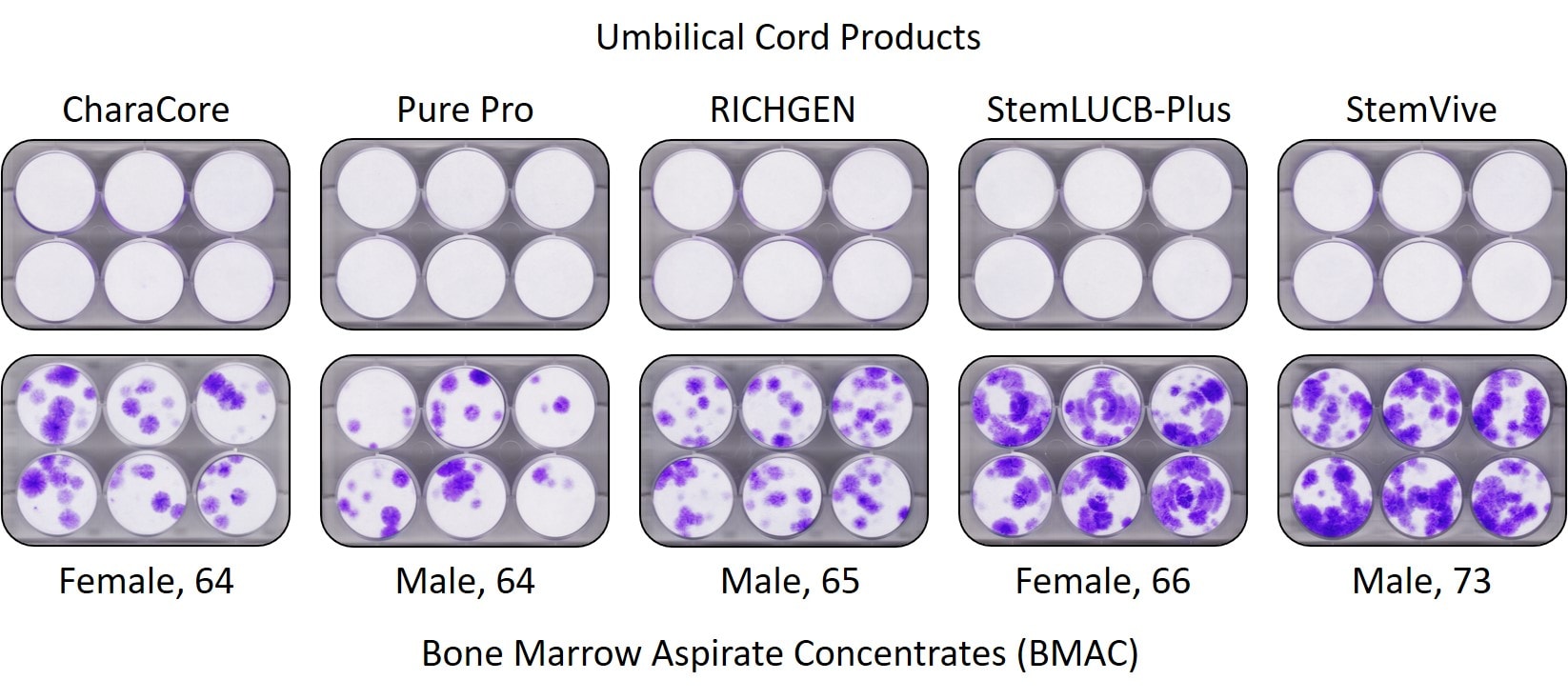
First, this is a CFU-f test. In this test, the product is placed onto these 6-well plates and then put into an incubator with media (cell food). The mesenchymal stem cells (as well as other cells) will attach to the plastic wells and then these can be stained. The attached cells (mesenchymal stem cells) turn purple here and the white means no stem cells. Note that none of the umbilical cord products we tested, including CharaCore, had any viable stem cells. This is in stark contrast to the stem cell content of the elderly bone marrow which is the second row.
You would think that this would be the end of CharaCore’s claims of selling a mesenchymal stem cell product. However, you would be dead wrong, as there’s MUCH more to this, in my opinion, sad saga.
The Altered Lab Report
A few months back, I was sent a lab report that purported to show that the CharaCore product had 20% by number of live and functional stem cells. I was intrigued given that two labs had found no stem cells, so I called and emailed the company that ran the tests for CharCore (BioArray). I sent them the report I had and this was what I asked and what was written in response:
- We were shocked reviewing the document you sent over! We have indeed done such a project back in August, but the data in the document you sent over are completely different from what we have in our record! However, we cannot send the original reports to you due to the agreement between the client and our company.”
- I was fine with not seeing the original report, so I asked about the viability and CFU numbers-“Unfortunately, that’s not what we concluded. The number of live cells per vial and their viability were much lower. The CFU test result was far below 99… I feel uncomfortable reading the numbers shown in the document because they failed to reflect the original data we obtained.”
- I then asked about the fact that the report concludes that 20% of the cells were MSCs-No, that’s not the wording of our report. Our conclusion read “The cells do not meet the minimal criteria of hMSCs.” In the report, we also concluded that the cells showed hepatic and fibroblastic differentiation. To be honest, I would suggest that you do not believe any numbers shown in that document. When I said they were completely different from our record, I meant “completely different”.
Meaning that what the report that was being freely circulated by CharaCore sales reps claimed and what the company that did the test had reported back to CharaCore was not consistent. At that point, I was certain that CharaCore would stop making these claims. However, I would again be wrong!
The Phoenix Rises from the Chared Ashes
I frankly hadn’t thought much about CharaCore as a result of the pandemic, but that changed this week when a colleague sent this Chara Biologics marketing email:
“Chara Biologics is the first in the industry to achieve a positive CFU assay result, demonstrating MSC colony formation-a definitive test for MSC presence and their viability and health…CharaCore is highly rich in MSC’s, where more than 20% of the total cell populations are MSCs, compared to most of the competitors’ 1% MSC content.”
There’s that pesky 20% MSCs claim again! That’s despite the company that actually produced that report reporting that this was false and not what they found.
What was sent as attachments were incredibly problematic, but let’s review:
- Claims of being 100% compliant with FDA, despite this letter sent to CharaCore about its non-compliance with FDA regulations.
- A testimonial of a traumatic brain injury patient treated with CharaCore, which is one of the things that FDA stated was not compliant (see FDA letter above).
- Claims of more than 2 million MSCs per ml of product! See the reality of n0 live and functional MSCs per ml as shown above.
- All sorts of claims that CharaCore has more stem cells than old people’s bone marrow, however see the pictures above. Elderly people had mesenchymal stem cells in their bone marrow, CharaCore had none.
What blew me away was the fact that Chara now has a sheet entitled, “Chara FDA Compliance and Third-Party Testing”. More astounding is that the same lab report I mentioned above from Creative Bioarray is still being used, even after a company representative informed me in writing that the conclusions in this report being circulated by CharaCore were not reported.
Putting CharaCore Claims in a Regulatory Context
These past few weeks have been interesting for providers who aren’t following the published research on these products that show that they have no mesenchymal stem cells. For example, we now have labs at CSU, Cornell, and UC Davis that show that these products have no live and viable stem cells (1-3). Hence, the FDA has stepped in and sent the following letters to doctors who didn’t get the memo:
- Henry Small, M.D. -An orthopedic surgeon in Houston who is treating Alzheimer’s, Lupus, Parkinson’s Disease, and Spinal Cord Injury with “cord blood stem cells”.
- Nilda A. Abellera, M.D.-Infuze MD of Milpitas, California who is using umbilical cord blood to treat spinal cord injury or damage, Parkinson’s disease, immunodeficiency disorders, and autoimmune diseases.
What’s interesting is that there is a distinct trend over the last two weeks. The focus of the FDA has shifted from just sending letters to the manufacturers of umbilical cord practices to focusing on doctors who are using them.
The upshot? At the end of the day, based on the data I have reviewed, there is no evidence that CharaCore is a stem cell product. There is concrete evidence that FDA doesn’t believe that CaharCore is compliant with its policies. There’s also mounting evidence, in my opinion, that the FDA is gearing up to stop a number of manufacturers and providers who are treating serious illnesses with umbilical cord “stem cells”. Hence, if I were Chara Biologics, I would be very worried about continuing to send out marketing emails like the one I reviewed.
_____________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.