Kimera Labs Exosomes Review
One of the newer, but actually old, concepts in regenerative medicine is exosomes. We have seen an explosion of interest in this product, but there is still very little clinical evidence that this approach works and even more regulatory uncertainty. Today I’ll review some recent communications I received from a sales rep hawking exosomes and take a look at all of these issues by reviewing the controversies.
What Are Exosomes?
To understand that better, watch my video below:
Basically, exosomes are little vesicles secreted by growing cells (including stem cells) that influence their environment and the cells around them. Why influence other cells? Repair is a complex symphony of different cells all acting in a coordinated manner. So, for example, some cells need to be told to become blood vessels and others to remove debris. Stem cells act as the conductor of that symphony.
Since the cells excrete the exosomes in response to what they detect is needed, exosomes are like the paint on a canvas. You can get art by randomly throwing paint on a canvas (think abstract art, like Jackson Pollock), but most art requires a brain that controls a hand that applies the paint. So exosomes without stem cells are like paint without an artist, as the cell coordinates which exosomes are needed and when.
How Are Exosomes Regulated?
What many companies are calling an exosome product is really conditioned media from growing mesenchymal stem cells that has been ultracentrifuged to concentrate small molecules. The media is the stuff in which the stem cells grow, and when the cells excrete exosomes and other chemicals into the media, it’s called a “conditioned media.” This isn’t a new or novel concept, as we’ve known for decades that the media in which stem cells grow can have effects on tissue, even without the cells.
Since getting the exosomes requires a culture of the cells, what is produced is an FDA-regulated cell drug that requires extensive clinical trials per medical indication. Specifically, I have confirmed many times with several regulatory experts that you can’t just register this product as a tissue through the 361 regulation process, which is a free online form to fill out. If you do that, then you have a misbranded and adulterated drug product. However, that seems to be what the companies selling exosomes have done. Want to learn more about the difference between a 361 registered tissue versus a 351 drug product? Watch my video below:
I even have direct experience in this area. We began culture expanding stem cells in 2005/6 and had to stop due to the FDA taking the position that this was a drug product that needed FDA approval. Sometime after that, I asked regulatory counsel if we could use conditioned media from the cultured cells since that would no longer be a “cell product.” I was told a hard and firm “NO” based on their opinion that this would constitute a drug product that would still require FDA approval.
A Ping on Facebook
Doctors get hit up all the time by sales reps. There are good sales reps and bad ones. The bad ones will say anything to sell a product. Usually, I’ll ignore these used car salesmen of the medical world, but every once in a while I get something that piques my interest. Here’s what I just got on Facebook:
“Good Morning! I am reaching via Facebook as I have been unable to reach anyone via phone and email. Regenexx came highly, highly recommended within the Regenerative Medicine Realm. I am the Global VP for Kimera Labs and we are the best of the best in exosome production. Our exosomes are 100% pure, FDA Cleared, ZERO resistance by patient..etc. I am aware that you guys perform an array of treatments. We have seen a 98% increase in the overall SUCCESS of Surgery, Treatment, etc in which Exosomes have been used. They can be used in PRP, in conjunction with Stem Cell or stand alone. They are 100% effective. An array of clinics, practices, hospitals etc are using them in conjunction with other procedures to ensure patient satisfaction and client retention. I would love to chat with someone regarding EXCLUSIVE pricing for REGENEXX! You do not want to miss out! AMAZING! You can reach me direct at 910..or via email at [email protected]”
An Interesting Phone Call
This woman provided her phone number, so I decided to call. I was told by her that the Kimera Labs exosome product has an “FDA Clearance.” This is interesting, as that regulatory language is only permitted to be used by a 510K cleared medical device where the FDA reviews the application. I tried to correct Amy several times, but she just kept saying that the product had been “FDA Cleared.” Why is this a problem? Kimera says its product is 361 registered as discussed above and the agency doesn’t “clear” or review this online form in any way. It’s merely a way for the FDA to come find a manufacturer if it wants to inspect the facility or recall a product. So right off the bat, Amy began by using misleading regulatory terminology giving the doctor the impression that the product had been reviewed and cleared by the FDA in some way.
I then asked Amy how she justified that the exosome product was 100% effective? She stated that this is what she heard from case studies (I presume from speaking with medical providers as she only has a master’s in biology). To find out more about all of this, I went to the website found in her e-mail address. The first thing I was required to do was to accept this disclaimer:
“Stemexcell.com is intended for properly licensed medical professionals only and makes no representation that any product(s) purchased are intended to treat, cure or prevent disease. An overview of the Frame Work for Regenerative Medicine Products from the FDA can be found here: https://www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/ucm585218.htm”
Huh? Your sales person just contacted me making very BOLD claims of 100% effectiveness for your product. She actually put that in writing!
Then we see this:
Hmm…I thought that the company made me click on a disclaimer that said that they weren’t making any claims about treating, preventing, or curing disease? Here it says that a football player got better with exosomes. Isn’t that a treatment claim?
Digging into Stemexcell.com
While a 361 registered tissue product is not permitted to make treatment claims, here’s what I found on this website as diseases that can be treated with exosomes (copy/pasted from the website):
- COPD
- Stroke-Following up with exome therapy immediately – in the first 36 to 48 hours – after stroke symptoms surface has proven to be crucial to long-term recovery and regaining mobility again. Cell therapy also calms post-stroke inflammation in the body, and reduces risk of serious infections.
- Parkinson’s Disease
- Hair Restoration-A thick, full head of hair is possible, naturally! Stem cell and exosome therapy promotes healing from within to naturally stimulate hair follicles, which encourages new hair growth. Using your own stem cells, Platelet Rich Plasma (PRP) and exosomes, you can regrow your own healthy, thick hair naturally – and restore your confidence!
- Erectile Dysfunction-Erectile Dysfunction (ED) is the inability to achieve or maintain an erection sufficient for satisfactory sexual intercourse. Regenerative medicine offers a non-surgical option that commonly uses the patient’s own stem cells, exosomes, and other sources of growth factors to regenerate healthy tissue to improve performance and sensation.
- Joint Pain-If chronic joint pain is derailing your active lifestyle, then you’re not alone. Regenerative medicine offers a non-surgical option that commonly uses the patient’s own stem cells, exosomes, and other sources of growth factors to reduce inflammation, promote natural healing and regenerate healthy tissue surrounding the joint for relief.
- Multiple Sclerosis
- Spinal Cord Injury-Spinal cord injuries are as complex as they are devastating. Today, cellular treatments, usually a combination of therapies, such as stem cell, Platelet Rich Plasma (PRP) and exosome therapy with growth factors are showing promise in contributing to spinal cord repair and reducing inflammation at the site of injury.
- Nerve Injuries-If you have chronic nerve injury pain that doesn’t fade, your health care provider may recommend surgery to reverse the damage. However, regenerative medicine offers a non-surgical option to repair damaged tissue and reduce inflammation at the site of injury. Stem cell therapy commonly uses the patient’s own stem cells, exosomes, and other sources of growth factors to regenerate healthy tissue.
- Neuropathy-If you have chronic nerve injury pain that doesn’t fade, your health care provider may recommend surgery to reverse the damage. However, regenerative medicine offers a non-surgical option to repair damaged tissue and reduce inflammation at the site of injury. Stem cell therapy commonly uses the patient’s own stem cells, exosomes, and other sources of growth factors to regenerate healthy tissue.
Exchanges on LinkedIn
I posted Amy’s sales ping on LinkedIn and was immediately attacked by Kimera’s national sales director. More interestingly was that he took the position that the company had no relationship with Amy. That was odd as she claimed to be the “Global VP of Sales” of the company. That also didn’t make much common sense as the Stemexcell.com website had the same logo as the Kimera website and many of the same stock and proprietary pictures.
Amy Is Fired by Kimera?
Next, the founder of the company, Duncan Ross, PhD, chimed in on LinkedIn that he had no control over what Amy was saying but that she would never again represent their product. On one hand, this is positive, as the founder is stating that he understands that what Amy said is over the line. On the other hand, the CEO of the company is directly responsible for what his contracted sales reps say. Meaning, that if the sales reps violate the law in selling his product, the buck stops with the CEO. Next, I then did some digging on the Kimera website.
Kimera’s Site
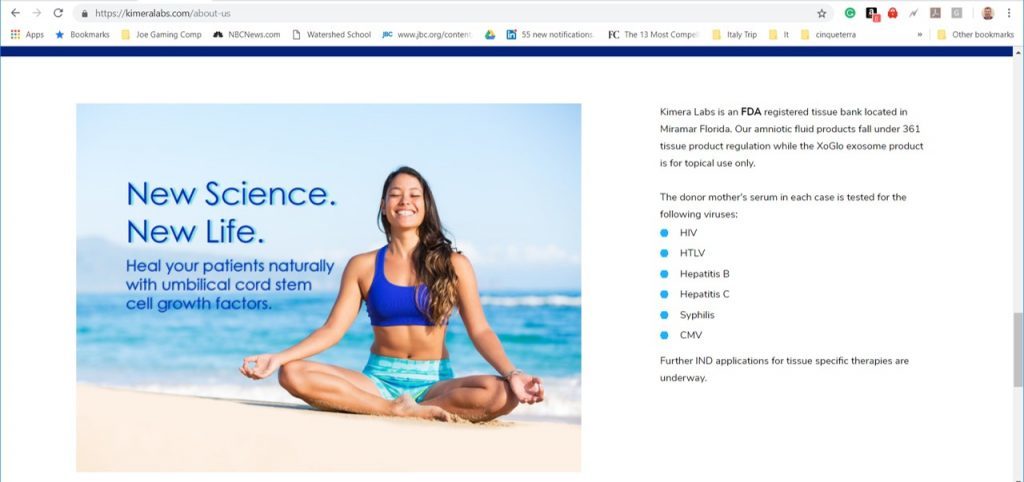
It looks like Kimera has two primary products they sell. One is a cosmetic creme containing MSC-conditioned media. The other seems to be amniotic fluid. Again, neither has an FDA cell-drug approval. Hence, neither is permitted to make treatment claims. However, I found this:
This is a treatment claim that states that you can heal your patients by using their product. For either product with a 361 registration, this would be beyond what is permitted by the FDA.
A Pending IND?
On LinkedIn, the Kimera Labs “US Director of Sales” claimed that in the next few weeks the company would be applying to the FDA for permission for a clinical trial. In the biologics world, that’s called a BLA/IND (Biologics License Application/Investigational New Drug). The problem is that while this makes sense, as exosomes are clearly a drug that requires FDA approval, the company is clearly selling this conditioned media product now, hence the aggressive marketing message that I got from their sales rep. To make sure that I was correct that a company that needs FDA approval for a product can’t sell that product before that approval is granted, I called an FDA regulatory legal expert. He confirmed that this was correct.
The position of the company is that it only sells the MSC-conditioned media drug as a cosmetic formulation. Does that get them a get out of jail free card? Not given the many FDA letters to cosmetics companies hawking similar products that also contained stem cell-conditioned media.
However, is there more to the story than exosome-laced cosmetics cremes? I and my colleagues have been bombarded for the last year or so with e-mails and private messages from reps who are purporting to sell the Kimera exosome product for orthopedic and neurologic use. As an example, I looked up an old e-mail ad that I was sent that asked me to attend a webinar put on by a sales distributor called Apex Biologics. This was found on YouTube, and it features Doug Spiel, MD, (medical director of Kimera Labs) hawking exosomes to treat neurologic disease. In fact, Doug had messaged me a year ago wanting me to use exosomes derived from stem cell-conditioned media and I told him that this product was an unapproved drug, so I passed.
So what proof is there that Kimera is selling not just the cosmetic version of the conditioned media but is also promoting it for other uses, like incurable neurologic diseases? This was found near the end of the video:
So on Black Friday, we could buy MSC-derived exosomes for $950 a vial! In context, the medical director of the company is telling me in this same video that I should use this product to treat incurable neurologic diseases.
Stemexcell.com Changes
Looks like Duncan Ross hammered the product distributor, as that website was quickly neutered to not say much of anything inside of 24 hours. I applaud Duncan for taking this action. I applaud the company for acting swiftly.
So Do Kimera’s Exosomes Work to Treat Orthopedic Diseases?
Right now we have little clinical data that this exosome drug product works to help common orthopedic issues. The www.stemexcell.com website lists research, but all of it is animal models or lab research. Some of this research doesn’t even have a direct relationship to clinical care. Meaning, there is not a single study listed that shows that the use of MSC-derived exosomes (conditioned media) works in real patients.
Any Comments from Kimera?
I reached out to both Adam Koster (US director of sales at Kimera Labs Inc) and Duncan Ross, PhD, (CEO/founder at Kimera Labs Inc) on LinkedIn for comment or any clarifications on either the treatment claims on the Kimera website or the Apex seminar. I got this response from Duncan:
“Thank you for bringing these issues to our attention. Please note that Ms. Nealey is not employed by Kimera.
Kimera Labs Inc does not condone and has never marketed our purified exosomes (not conditioned media) for the use of neurological diseases. We shared our wound healing marketing materials with the FDA when they audited us on Nov. 24, 2017 and have not changed them since.
We also do not control what physicians do with our product, what they teach, or what representatives disseminate without our permission. We are investigating the claims that you raised and are trying to improve training for our distributors.”
The upshot? It’s a full-time job keeping up with this stuff. It’s hard to believe that in 2019 we have orthopedic sales reps who are silly enough to claim that a nonapproved and FDA-regulated drug product with no published clinical research has 100% efficacy. It’s also amazing to me that we have a company that is selling a culture-expanded drug product without FDA approval.
On a personal note, why take the time to write this stuff? Because as a thought leader in interventional orthopedics and orthobiologics, I get asked questions about these products all the time. It’s far easier to take the time to dig deep and then send a link to this blog than to explain this all a hundred times to a hundred different doctors or patients!

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.