Should Sales Reps Vet the Products They Sell? Apex Biologix
Does a medical distributorship have a responsibility to vet the products they sell to physicians? Or is this an arm’s length relationship and buyer beware? Today, let’s view this controversy through the lens of some of the products pushed by a medical sales company in the regenerative medicine space called Apex Biologix. Let’s get started.
The Controversy
Physicians often rely on medical sales reps to provide them with education on new topics. While it’s usually a bad idea to rely on someone selling you something as a voice of reason, this exchange of information still happens countless times a day. Let’s explore the issues around these educational-sales relationships.
If the information conveyed by a salesperson is accurate and balanced, then a sales rep should be able to educate a doctor without issue. However, if the information is inaccurate and biased, then the public should be concerned. In addition. if this is where the doctor gets most of his education on that one topic, this educational sales encounter could cause real harm.
Hence, the public should be less concerned about educational sales encounters if the company selling the product has done high-level due diligence on what it’s selling and more concerned if there is no or little due diligence. Let’s explore this thorny topic through one example in the new regenerative medicine space.
Our Example: Apex Biologix
Apex is a medical distributorship. What’s that? They act as a middleman between a product manufacturer and doctors. This allows the manufacturers to spend less on marketing as these distributorships usually have a team of commissioned salespeople.

The only name associated with the company that I recognized was Dan Crane, an MBA who is associated with a slew of businesses. He is co-founder of Apex Biologix. He is the CEO of a medical management, consulting, and billing firm (CE Medical Group). Also, he is on the executive team of the “Advanced Regenerative Medicine Institute” (ARMI). Based on his Linkedin profile, his background prior to 2014 seems to be focused on medical billing, staffing, and home health services.
What is ARMI? It seems to be the for-profit training arm of Apex Biologix. Why would a medical distributorship have an education arm? This has become increasingly popular of late as these distributorships seek to increase their market share in a world where most physicians still don’t know which end is up with regenerative medicine. The problem is that this trend also means that most regenerative medical education is not being delivered by non-profit physician groups, but instead by for-profit companies that are pushing specific products.
When I dig deeper on the ARMI page, I found a “Medical Team”. Let’s dig in there.
The “Medical Team”
So who is the “Medical Team”? In my opinion, that phrase clearly relays that there is some sort of advisory and collaborative relationship between the business people at this medical middle man company (aka distributorship) and the doctors listed. Several physicians are listed, some of whom I know personally so I reached out to them. Were they actually advisors to Apex? No. One didn’t know why they were listed on the site and the other two had taught some courses for the company. So much for a “Medical Team”.
Apex and the Scam of the Century
One of the biggest medical scams of the 21st century has been companies selling umbilical cord “stem cells”. Regrettably, Apex Biologix was right in the middle of that push to have physicians believe that the umbilical cord products they were selling contained live and functional stem cells. They’re also still doing this on their website today. Let’s dig in.
Several years ago, multiple umbilical cord tissue processors appeared on the scene with new and different messaging. A good number claimed that their frozen products made from parts of the birth sac or umbilical cord contained many live, young, and functional stem cells. Some medical distributorships and gullible physicians did little to check the veracity of these claims. Meaning they bought the song and dance from the companies manufacturing these products hook, line, and sinker. This ball of fiction rolling downhill began one of the biggest medical scams since Laetrile.
Apex Biologix was right in the middle of that sale. In fact, I blogged on a 2018 marketing email that Apex sent to physicians hawking StemVive and StemShot (Utah Cord Bank products) as containing live stem cells.In my opinion, any actual expert in Regenerative Medicine could have easily seen that what the Utah Cord Bank was claiming was not supported by the company’s marketing materials.
For example, in a March 23rd, 2018 marketing email Apex claimed: “StemVive® provides a stem cell-based product intended to promote tissue regeneration and healing. ” Later, we tested StemVive in our lab and had the CSU Translational Medicine Institute test a second sample. The results are below:
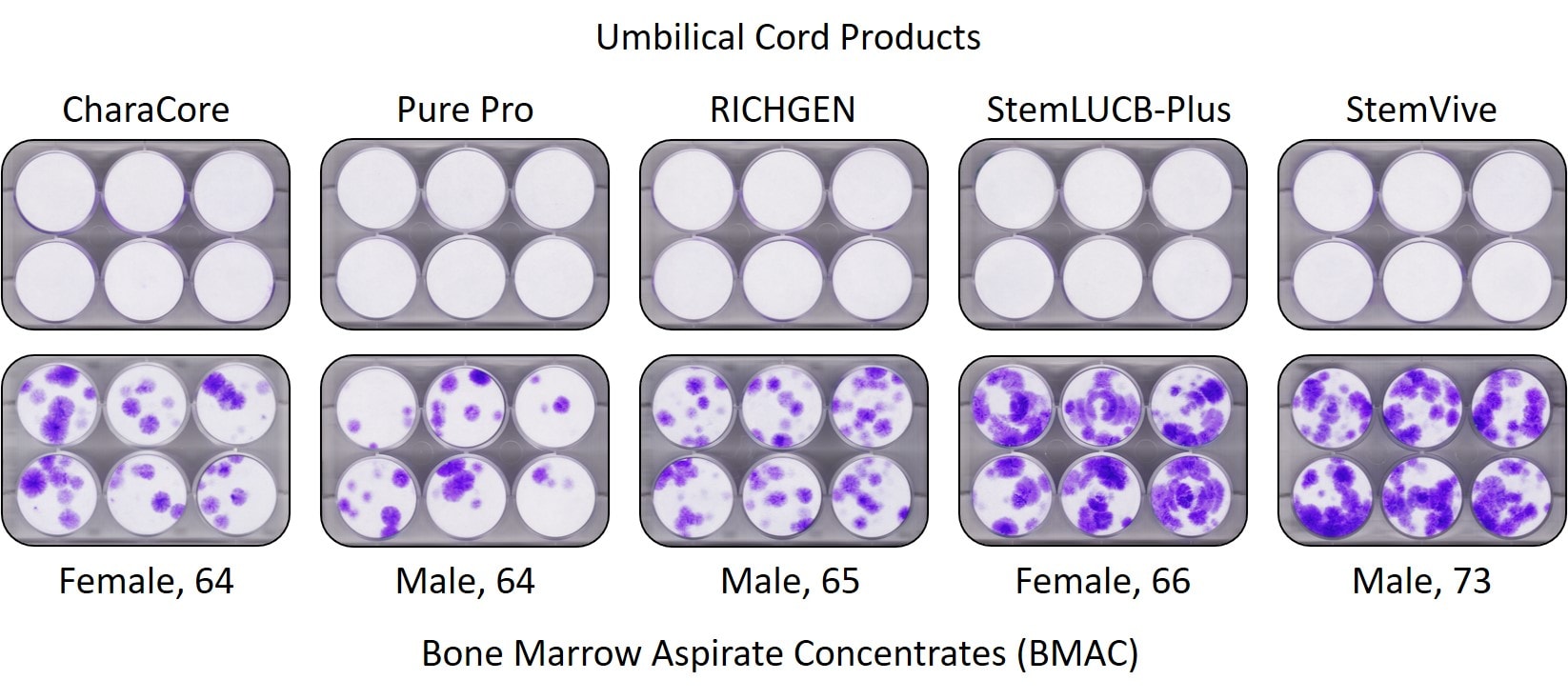
Note that the StemVive sample that Apex claimed had live stem cells failed this simple CFU-f test (far right and on the top row above). The all-white you see in those StemVive test wells means no mesenchymal stem cells were obtained from this test. In fact, if you compare that to the stem cell content of the 73-year-old male below, where the purple dots denote stem cell colonies, the stem cells in this elderly man’s bone marrow blew away the Apex product.
Is this the only study showing that birth tissues have no stem cells? Nope. Several university labs have studied this issue as well and also found no living stem cells (1-3).
What Duty Does a Middleman or Physician Have in Performing Due Diligence on Products?
So what duty did Apex have to the physicians who received these emails? Did they have a duty to at least vet the claims of the company it represented with actual published stem cell experts? I confronted one of the physicians teaching Apex courses back then with the mounting evidence that what Utah Cord Bank was selling wasn’t as advertised. It was clear to me at that time that this physician didn’t have a basic understanding of how to vet the scientific claims. This wasn’t his fault, very few physicians have published research in this area or have any deep understanding of the science.
Should Apex also have sent the products that it was selling for basic independent lab tests with labs not associated with the Utah Cord Bank? That would have cost at most $10,000 to get the test shown above done. For a product that sells for more than a thousand dollars for a 1-2 ml vial, in my opinion, that’s not much to spend to ensure that what you’re telling your customers is accurate.
Maybe Apex Just Had this One Little Issue?
Since we tested many of the amniotic and umbilical cord products that were being hawked to us in our state of the art lab to ultimately find out that the manufacturers weren’t being honest about what their products contained, maybe Apex has just had this one little lapse of judgment by not paying for those tests? I wish that were the case, but check out this November 2018 marketing email sent to my physician colleagues:

Here Apex is now selling exosomes. Let’s dig in there to see why this is a problem.
What is an Exosome?
Exosomes are produced by all cells of your body as a communication vehicle. They are small vesicles that can carry any number of messages. To learn more, see my video below:
There are three serious problems with physicians using exosomes right now:
- We have zero evidence that exosome products help patients.
- We have no way to isolate only the good exosome messages that may help patients and separate them from the bad exosome messages that may hurt patients.
- Selling exosomes without full FDA drug approval after successful clinical trials is clearly illegal.
So again, here we have another due diligence issue. For example, many years ago, our company vetted whether selling the media that grew stem cells (which is what Apex is selling here) would be legal. The opinion from regulatory counsel came back that this would NOT be legal. Why didn’t Apex pay for that same legal opinion here? Again, we’re talking about a $10-20,000 legal opinion from a qualified regulatory attorney for a product that sells for a cool thousand dollars a vial.
The Umbilical Cord and Exosome House of Cards Collapses
While a qualified regulatory attorney could have told you in 2018 that selling a vial of umbilical cord “stem cells” or exosomes manufactured by growing mesenchymal stem cells required full FDA approval and clinical trials for each clinical indication, the FDA itself decided to step in a year later. It issued this unprecedented public warning:

This warning to middlemen like Apex and many others put all on notice that they were NOT permitted to sell stem cell or exosome products. The FDA warned that there were no approved exosome products and that claiming to sell a vial of stem cells or exosomes without clinical trials and evaluation by the FDA as a new drug was illegal. So maybe Apex again just had these two slip-ups? Let’s now meet Organicell.
Apex and Organicell
Recently Apex sent out a marketing email that discussed a webinar on the use of exosomes produced by a company called Organicell. Huh? Apex and others were informed via a public warning in late 2019 that to sell exosome products would require a cellular drug approval per clinical indication. That means that given that no exosome product on the market has that kind of approval, that selling any exosome product right now is NOT legal.
I went to the Apex website and found a chatbox application. Remember as you read this chat transcript from that visit that no one is permitted, by federal law, to sell exosomes or to claim that they will heal any condition, like knee arthritis:
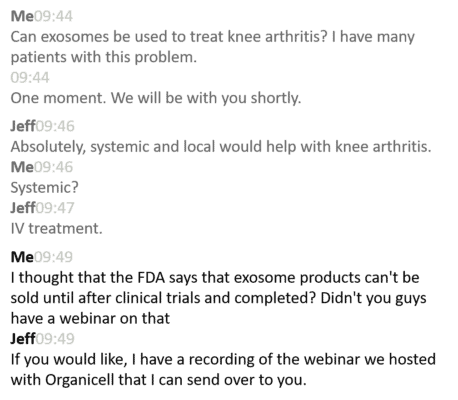
So here I am being told by a sales rep for Apex that exosomes can treat knee arthritis. Do we have a single clinical study that proves this? No. In fact, I’m being told that I don’t even need to inject them in the knee, just give the exosome product through an IV! Is there any clinical research supporting this? Nope.
Jeff then went on to state that Organicell had gotten FDA approval for a clinical trial in end-stage COVID-19 patients. Remember that this is not an FDA approval to sell their product, but only an emergency authorization during this pandemic to conduct a study on this one topic. I then asked if they had any FDA approvals to study or treat knee arthritis. The answer was “No”.
The Organicell Webinar
I watched the webinar sponsored by Apex that was put on by Organicell. Remember again that promoting exosomes for the treatment of any condition is not legal at this point. On the webinar, Organicell promoted using exosomes for treating hair loss and facial cosmetic use. During the Q and A, Organicell staff discussed using the product to treat osteoarthritis including the hip. The staff even made dosing recommendations, positing that hip arthritis requires a higher exosome dose. The company CEO also discussed that using IV exosomes can impart a neurologic benefit. I won’t go into much more, but suffice it to say that many treatment recommendations were made including offering physicians specific treatment protocols that in my opinion, were currently illegal per FDA regulations.
The 361 Two-Step
The company CEO on the webinar used what I call the 361 two-step to make it appear that his exosome product is not regulated as a drug. This technique involves claiming that since this is merely ultra-centrifuged amniotic fluid tissue, that the product can be sold as a transplant tissue without FDA approval, but with merely a 45-minute quickie tissue registration (361). The problem with that idea is that the FDA system is a claims made process. Let’s dig in.
A great example the agency gives is selling charcoal. You can sell charcoal for non-medical uses like heating a BBQ. However, the minute you begin to claim that charcoal can be used to treat a disease (like poisoning), your charcoal becomes a drug that requires FDA approval. The charcoal may be the same product, but it’s the claim that drives the regulation.
Hence, when the CEO of Organicell began using the term exosomes and putting on a webinar where medical treatments are discussed, he made the product a drug requiring full FDA approval and clinical trials for every indication he or his staff reviewed. So when the physician he employs discussed using the product to treat hip arthritis, the company would need to pursue years of clinical trials for that indication (hip arthritis) and have those results reviewed and approved by FDA. The quickie 361 registration is now the WRONG regulatory pathway. To learn more on this 361 FDA tissue registration issue, watch my video below:
What’s on the Apex Website Today?
Maybe Apex has cleaned up some of its act through the years? After all, for example, we now have many lab studies showing that birth tissue products contain no live and functional stem cells. We also know through the FDA public warning that you can’t claim to sell an umbilical cord stem cell product without a drug approval. Maybe they got that memo? Here’s what they have on their website of this morning on the Apex website
“Derived from birth tissue donated by mothers delivering healthy live birthed babies by Caesarean section, StemVive™ provides a stem cell-based therapeutic product intended to promote tissue regeneration and healing.”
I guess Apex still hasn’t gotten the memo…
The upshot? Does a medical distributorship have a responsibility to vet the products they sell? The regen med space has been out of control in this regard and in my opinion, this wild west begins with medical sales companies. Apex Biologix is just one example of many. Again, in my opinion, they have had a consistent and clear pattern of either selling products that it didn’t vet or test like StemVive or products that can’t be legally sold the way they are advertised. That last part is clearly the case in claiming that StemVive is a stem cell product, or selling exosomes derived from culture media, or even as of last week, allowing one of its new exosome vendors (Organicell) to make treatment claims in a public forum promoted by Apex. So if as physician leaders we’re going to clean up regen med, that starts with calling out medical distributors who are adding to the carnival atmosphere.
________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
