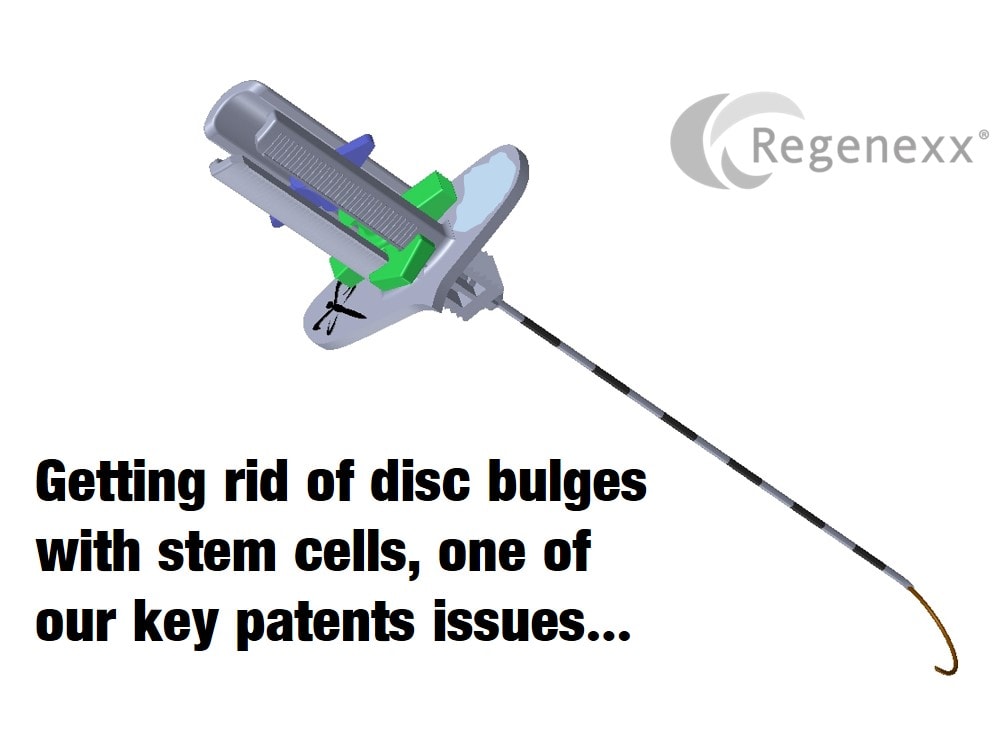
Back Pain Stem Cells: A Key Regenexx Patent Issues
We’ve been treating low back discs with stem cells for about a decade. In all of that time, we realized that there was a need for a device that would allow us to place stem cells into parts of the disc that traditional needles couldn’t reach. As a result we designed the device and submitted a patent and this year the USPTO granted it. The story of how that came to be says a lot about back pain stem cells and what they’re capable of and what they can’t do.
For background, our low back disc bulge stem cell technology is very unique. Early on, before any pharma cell companies had thrown their hat into the low back disc stem cell treatment ring, we realized that the amazing things that rabbit animal models predicted weren’t necessarily happening in humans. For example, you could regrow a disc almost new after destroying it in a rabbit, mouse, or dog by simply injecting stem cells. When we replicated this in 2006 and 2007 in real patients, those with severely degenerated discs didn’t regrow their disc like new. This caused us to go back to the proverbial drawing board and try to rethink the problem.
Around 2008 or so, we realized that the idea of regrowing severely degenerated discs was a non-starter in humans. So we focused on what was achievable with stem cells-getting rid of a disc bulge that was pressing on a nerve-something that was a huge problem for millions of patients every year who needed back surgery to cut out the offending disc. We made several changes to the process to get this to work including specialized stem cell culture conditions to mimic the harsh environment of the disc and placing the cells into certain hard to reach areas of the structure. At that point we began to see great results in patients with disc bulges using this specialized stem cell technique. Since then we’ve refined other techniques using same day stem cells to help heal a disc tear.
A number of years ago we licensed our cultured stem cell disc bulge treatment to a publicly traded company now called Biorestorative Therapies (BRTX). That company has since begun the process of FDA approval and should start phase 1 clinical trials soon. They announced this week that a patent had been granted on our disc device (press release below).
The upshot? The companies now trying to get FDA approval for an off the shelf vial of injectable stem cells to regrow discs are finding out the hard way that this doesn’t work so well. Thankfully we figured that out long before many of those companies were ever founded. BRTX has licensed a low back disc stem cell technology that we have seen time and time again work well, so hopefully they will be able to push the FDA rock uphill!
The Regenexx-C procedure is not approved by the US FDA and is only offered in countries via license where culture expanded autologous cells are permitted via local regulations.
______________________________________
BioRestorative Therapies Announces Patent Granted for Its Licensed Curved Needle Device
MELVILLE, N.Y., Nov. 3, 2015 (GLOBE NEWSWIRE) — BioRestorative Therapies, Inc. (“BRT” or the “Company”) (OTCQB:BRTX), a life sciences company focused on stem cell-based therapies, today announced that the Company is the beneficiary of a patent granted for a licensed curved needle device (“CND”) designed to deliver cells and/or other therapeutic products or material to a site having damage in need of facilitated repair.
The claims of the patent relate to devices and methods for optimized or facilitated delivery of therapeutics to a target site in a patient in need of a biologic therapeutic. The Company intends to advance the design of this curved needle device to facilitate the delivery of substances, including living cells, to specific locations within the body and minimize the potential for damage to nearby structures.
The device relies on the use of pre-curved nested cannulae that allow cellular and other therapeutics to be deposited in areas where direct access is not possible due to outlying structures (e.g., vertebra, spinal cord and spinal nerves with regard to the disc).
The therapeutic delivery device patent was issued to BRT’s licensor, Regenerative Sciences, LLC (“Regenerative”), on August 25, 2015. Pursuant to a license agreement, BRT has obtained a worldwide, exclusive, royalty-bearing license from Regenerative to utilize or sublicense the medical device for, among other purposes, the administration of specific cells and/or cell products to the disc and/or spine (and other parts of the body).
About BioRestorative Therapies, Inc.
BioRestorative Therapies, Inc. (www.biorestorative.com) develops therapeutic products and medical therapies using cell and tissue protocols, primarily involving adult stem cells. Our two core programs, as described below, relate to the treatment of disc/spine disease and metabolic disorders:
• Disc/Spine Program: Our lead cell therapy candidate, brtxDISC™ (Disc Implanted Stem Cells), is a product formulated from autologous (or a person’s own) cultured mesenchymal stem cells collected from the patient’s bone marrow. We intend that the product will be used for the non-surgical treatment of protruding and bulging lumbar discs in patients suffering from chronic lumbar disc disease. The treatment involves collecting a patient’s own stem cells, culturing and cryopreserving the cells, and then having a physician inject brtxDISC™ into the patient’s damaged disc in an outpatient procedure. The treatment is intended for patients whose pain has not been alleviated by non-invasive procedures and who potentially face the prospect of surgery.
• Metabolic Program (ThermoStem®): We are developing an allogeneic cell-based therapy to target obesity and metabolic disorders using brown adipose (fat) derived stem cells to generate brown adipose tissue (“BAT”). BAT is intended to mimic naturally occurring brown adipose depots that regulate metabolic homeostasis in humans. Initial preclinical research indicates that increased amounts of brown fat in the body may be responsible for additional caloric burning as well as reduced glucose and lipid levels. Researchers have found that people with higher levels of brown fat may have a reduced risk for obesity and diabetes. The Company is a party to a research agreement with Pfizer with regard to the study of brown fat.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. You are cautioned that such statements are subject to a multitude of risks and uncertainties that could cause future circumstances, events or results to differ materially from those projected in the forward-looking statements as a result of various factors and other risks, including those set forth in the Company’s Form 10-K filed with the Securities and Exchange Commission. You should consider these factors in evaluating the forward-looking statements included herein, and not place undue reliance on such statements. The forward-looking statements in this release are made as of the date hereof and the Company undertakes no obligation to update such statements.
CONTACT: Lee Roth / Joseph GreenThe Ruth Group for BioRestorative Therapies
646-536-7012 / 7013
[email protected] / [email protected]Source: BioRestorative Therapies

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.