BioGenix Review
I literally can’t keep up with the clinics and vendors pushing umbilical cord products. This morning’s entry is BioGenix. Who is that? Let’s review.
Umbilical Cord Products
You can’t attend a seminar these days without being told that the umbilical cord product they’re using has millions of live and young stem cells. Is that true? Let’s dig in.
Unlike a patient who hears this and just believes what they’re told, I have a bigger responsibility. Our clinic in Colorado which serves as Regenexx HQ has a million dollars worth of lab equipment and a Ph.D. lead staff that can get to the truth of almost any claim about stem cells. Hence, when I was first approached about a birth tissue product containing millions of stem cells in 2014, I didn’t immediately trust the sales rep selling the product. We tested it. I frankly wanted to believe, like many patients do, that what this guy was telling me was true. However, our lab tests were clear, no living stem cells. That first test some 5 years ago began the odyssey that leads me to this blog on Biogenix.
What Greets You at the Biogenix Website
Many websites selling medical products have a disclaimer, but the BioGenix disclaimer is special:
The first part is pretty typical, but then there are numerous references to this being a mesenchymal stem cell therapy product. This is not an FDA compliant disclaimer, how do I know that? You need a full FDA 351 drug approval to claim that your product has mesenchymal stem cells and Biogenix is telling us here is has no such FDA approval. In fact, the disclaimer takes the language from a series of FDA statements warning about companies selling “stem cells” and turns it 360 degrees. If you want to understand why BioGenix claiming that its product has MSCs makes it an unapproved drug that can’t be sold as a tissue registered by this company, watch my video below:
Digging Further In…
On the front page, BioGenix claims to be the world’s leading regenerative medicine company. That’s a really big claim. If that were the case we would expect to see certain things:
- A decades-long track record
- A huge pharma like operation
- Lots of scientific research using its product
- A massive science team and research output
A Decades-Long Track Record: A great way to research this is through the Internet Archive. The company lists it’s founding in 2016 on Linkedin and puts up a website sometime in late 2017. Hence, it’s been open for business for less than 24 months.
A Huge Pharma Like Operation: The company claims in it’s Linkedin profile to have 200-500 employees, so what’s actually on Linkedin? As far as employees that work full time for the company, there are 13 in the US listed on Linkedin (the company is based in Houston, Texas). If we compare that to Pfizer, they have 130,000 employees listed on Linkedin. If we dive into the 13 employees for BioGenix, most are sales reps. I dove into one profile (Kevin Cummings) who has been a territory manager for the last year. He worked at Orkin as an account manager prior to BioGenix. Mike Stavikski is listed as a Vice President, he was at Liveyon (the company that had an umbilical cord contamination problem sending a dozen patients to the ICU) and before that, he was in medical sales. Hence, this is a new company with a handful of salespeople as employees.
Lots of Scientific Research:
I ran a search at the US library of medicine and found no research studies performed on this product. Kevin Cummings’ Linkedin profile claims that BioGenix has more than 120 indications! The website says that the product can be used in lots of clinical indications, so let’s dive into one-Orthopedics. The research listed is the study at this link. In one of the tables in the linked research paper, 15 papers looking at animal models of cartilage repair through mesenchymal stem cells are listed. How many used BioGenix as a product. Zero. How many used umbilical cord derived MSCs, zero. In fact, almost all of the research listed is on bone marrow derived MSCs.
A Massive Science Team: There are no scientists that work for the company listed on its website or Linkedin. Think about that for a second. This company claims to be the world’s leading regenerative medicine company. That’s a field in biotechnology, hence, we should see a huge science team. In fact, their website states that they are working with a lab partner, which means they are likely private labeling someone else’s product. This is confirmed by their FDA Tissue Establishment Registration, where they are listed not as a manufacturer of any product, but simply as a product distributor.
MSCs in Umbilical Cord Products
So it’s clear that BioGenix is a small sales operation that began selling umbilical cord “stem cells” less than 24 months ago. It has no clinical research on its product. The only research it does list has nothing to do with its product. I has no research team toiling away at their lab benches. In fact, it manufacturers no product, but simply distributes one made by someone else.
Since it has the phrase “mesenchymal stem cells” littered all over its website, what is the likelihood that this umbilical cord product has any live and viable MSCs? Let’s look at the test plate we just got back from the Translational Medicine Institute at CSU. They tested five commonly used umbilical cord products versus middle-aged and elderly bone marrow:
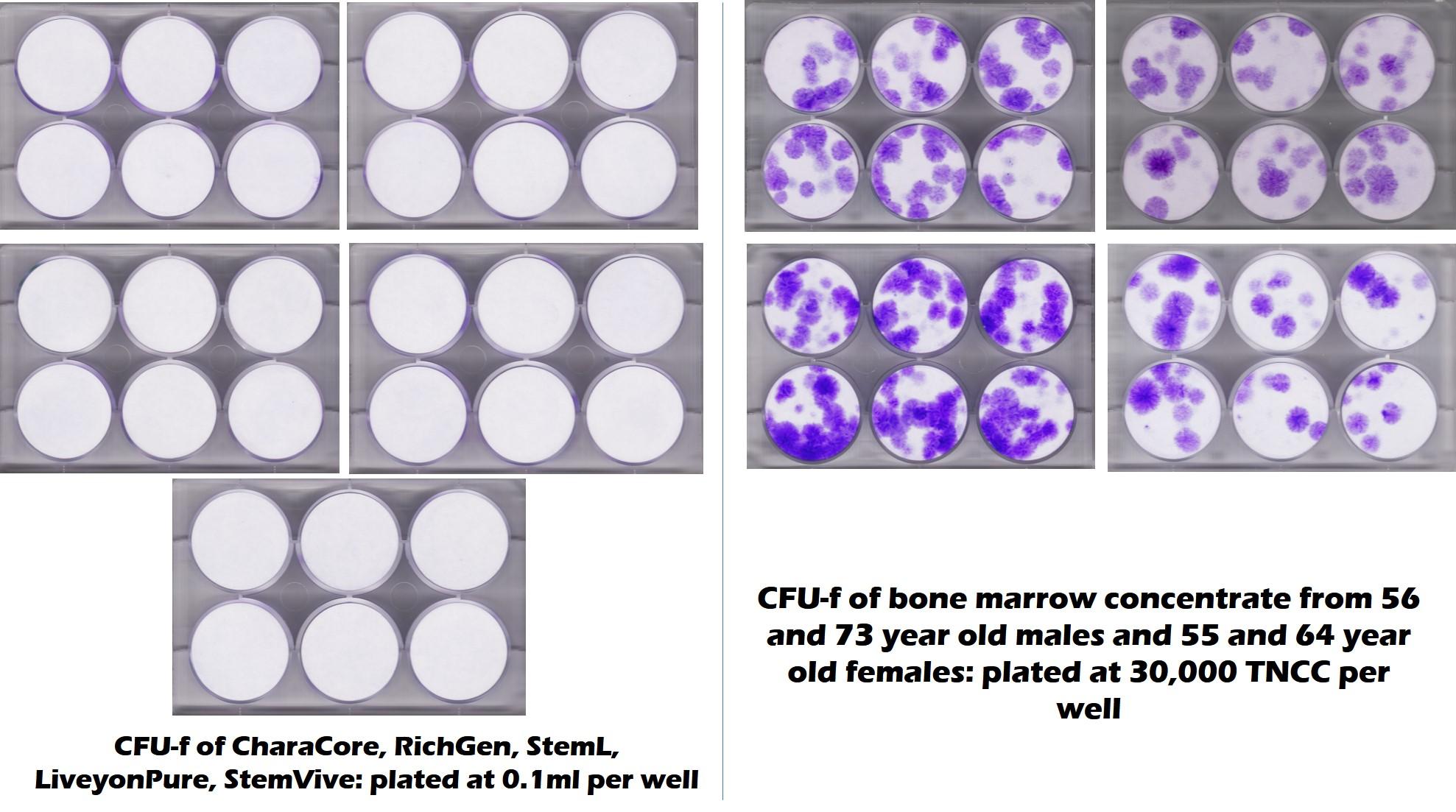
The purple dots here represent stem cell colonies. All five of the umbilical cord products on the left have no mesenchymal stem cells compared to the many found in the middle-aged and elderly bone marrow. So what’s the likelihood that the BioGenix umbilical cord product has stem cells? Slim and none and Slim is literally on vacation in Texas (Houston to be exact). Others have published the same results on amniotic and umbilical cord products (1-3).
The upshot? In my expert opinion, after reviewing everything on BioGenix, this is a small sales operation and not a biotechnology company nor a stem cell product. Please do your homework before you get any treatment!
_______________________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
