Burst Biologics Gets and FDA Warning Letter: Claims vs Reality

Yet another Umbilical Cord product manufacturer has received yet another FDA letter. This time it’s Burst Biologics. I thought today that I would use this as an opportunity to show you the difference between what you can find on the web against the reality of what an FDA inspection found on the ground at the company processing site for Burst. Let’s jump in.
What Is Umbilical Cord Blood?
Umbilical cords have blood inside them and this has been approved for use by the FDA to treat pediatric blood cancers. However, there is no other FDA approval for umbilical cord blood, so if the agency catches you selling it for any other purpose, it declares that product an unapproved drug. Many companies try to get around this need for extensive clinical trials by registering their product as a tissue which is a free quickie website form.
An Examination of the Claims Made by Burst Biologics vs. What Was Found in their Recent FDA Inspection
I reviewed what was on the Burst Biologics website as of this morning versus what was in their recent FDA letter. Here’s what I found:
Regulatory
Burst claims on its website that its products are 361 registered tissues. That means that the company only has to go through a free 45-minute online registration and doesn’t need to spend the hundreds of millions and 5-10 years it would normally take to get a new drug approval.
This is what’s on the company website:

This verbiage from Burst Biologic’s regulatory section of their site states that the company registered its tissue as a 361 and that according to the manufacturer, they don’t consider this product a drug that would require extensive clinical trials. What did the FDA think about that in the recent Warning Letter it sent Burst?
“Your products are also human cells, tissues, or cellular or tissue-based products (HCT/Ps) as defined in 21 CFR 1271.3(d) and are subject to regulation under 21 CFR Part 1271, issued under the authority of section 361 of the PHS Act [42 U.S.C. 264]. HCT/Ps that do not meet all the criteria in 21 CFR 1271.10(a), and when no exception in 21 CFR 1271.15 applies, are not regulated solely under section 361 of the PHS Act [42 U.S.C. 264] and the regulations in 21 CFR Part 1271. Such products are regulated as drugs, devices, and/or biological products under the FD&C Act and/or the PHS Act, and are subject to additional regulation, including appropriate premarket review.”
What the heck does all of that mean? Basically, the FDA considers the Burst Biologics products an unapproved drug.
Score 0 for Burst Biologics and 1 for reality.
Quality
This is what the Burst website says about product quality:
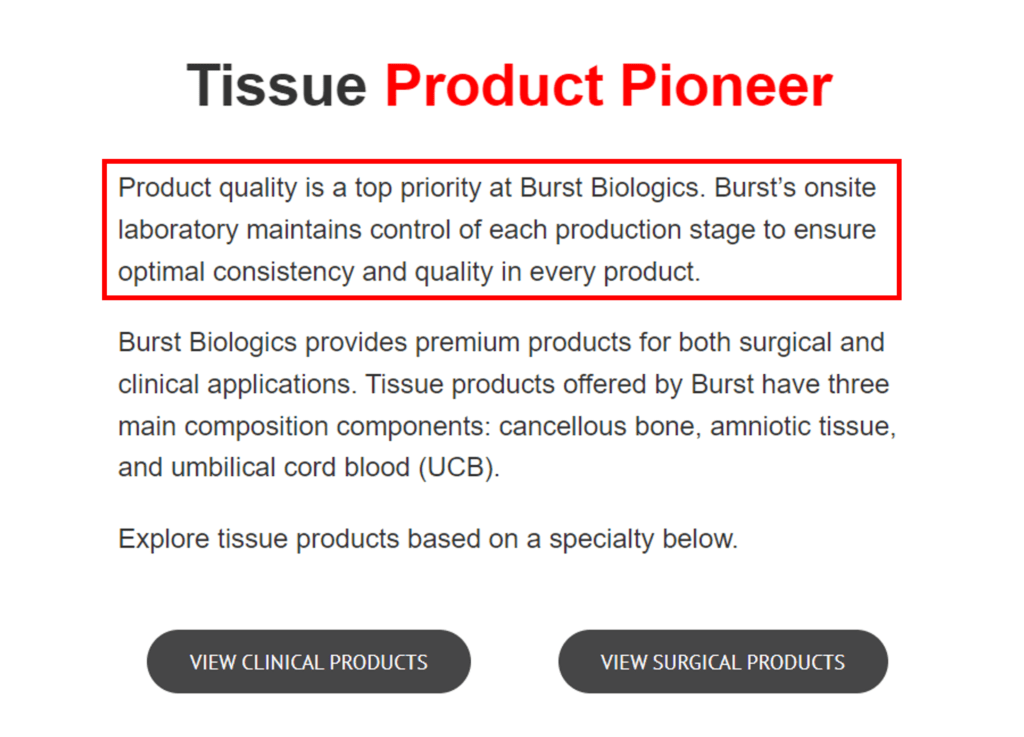

We see two references above to quality and strict oversight of the production process. What did FDA think?
Here were the quality problems noted by the FDA inspection:
- A failure to properly screen donors for communicable diseases. In this case a failure in 2016 to test donors for the Zika virus.
- No written SOPs that cover all aspects of sterile processing. This included not using what’s called a “processing blank” or not using it up to FDA standards. This is where processors are tested for their sterile handling by processing just cell culture media that is used which is then tested to then see it grows bacteria. In this case either no media fill (processing blank) was used or when it was used, the company failed to incubate it enough to actually grow out microorganisms. The FDA was upset here because all of this product made during this time was sold and shipped and likely ended up in patients.
- The company failed to properly monitor the workspaces that were in the processing lab by allowing too many bacteria to grow in test plates taken from this area and from the PPE worn by processors. Basically, the company allowed too much contamination in its tests, which the FDA believes poses a “significant safety concern”.
- A failure to test all of the components used in processing for sterility before using them.
- A failure to investigate breaches of sterility that had happened during processing to determine where the contamination originated.
It looks like the company tried to write two letters addressing the FDA’s concerns that the agency found to be inadequate.
Summary
The FDA is sticking to its guns that the Burst product is in fact an unapproved drug. Hence, no matter how these safety concerns are addressed, the product needs to go through full FDA approval with clinical trials BEFORE it can continue to be sold on the market.
The Aircraft Carrier
For those of you wondering where all of the letters are for the various Umbilical Cord, Exosome, and Amniotic products that decided to stay on the market as of the end of the FDA Discretionary period last year, this Warning Letter holds some clues. These inspections took place about a year ago and we’re just seeing this letter. You can bet that many of these companies still selling these products also got inspections. How do I know that? Some have begun hiring attorneys in a very public way and making statements that they are not drugs. IMHO, you can bet a large sum of money on the fact that this means that they have been inspected already and failed in a similar way to Burst Biologics. However, given how slow the process moves, we likely won’t see these letters until late this year at the earliest or even next year.
The upshot? The Umbilical Cord products made by Burst Biologics are an unapproved drug according to FDA who also found all sorts of safety issues at the processing site. This letter to Burst is likely one of a half dozen or more that we’ll see issued this year and next. Just realize that the process of inspection and then reinspection and then back and forth with the company takes a long time.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
