LifeVault and Forever Labs: Will This Insurance Ever Pay Off?
We buy insurance because we all want to make sure that if something awful happens, we are prepared. Many years ago I was asked to be on the scientific advisory board of a company that was collecting stem cells from patients for future use. The venture never really worked out, primarily because this was like buying insurance when it was unlikely to pay out. Let me explain.
Why Write?
Why do I take the time to do these blogs? The big reason is that I get inundated with e-mails, patient in clinic questions, and online queries about each and every technology on which I blog. Rather than going through a detailed 20-minute lecture each time, it’s easier to review something once and place my analysis online. That way, I can just send someone a link.
My “Stem Cell” Insurance Experience
The company I worked with used a mobilized peripheral blood apheresis protocol. This meant that they gave the patient a drug to knock stem cells loose from the bone marrow and into the bloodstream. They then used a machine process called apheresis to collect a cell fraction from the blood that would hopefully contain those cells and then deliver the rest of the blood product back to the patient. At one point we were asked if we could grow stem cells from their apheresis product. Regrettably, no mesenchymal stem cells grew, so we concluded that whatever stem cells they were collecting were “barely there.”
The company also had a bigger issue. They had a hard time selling patients on these collections because the cells they stored couldn’t legally be used in the U.S. to treat anything. So this was like buying an expensive insurance policy that would never pay off.
Are There Stem Cells in Blood?
We see many physicians making the claim that they are performing “stem cell therapy” merely by collecting a blood sample. Regrettably, this is mostly fiction as blood is very poor in any active stem cell content. In fact, what the patient is really getting is a hyperexpensive platelet-rich plasma therapy.
LifeVault
The process behind LifeVault sounds intriguing. You send in a blood sample, and according to their website, they separate the blood sample into “DNA,” “Stem Cells,” and “Blood Plasma.” The site then claims (as an incentive to save your blood sample) that stem cells are being used currently in research studies to treat ALS, multiple sclerosis, Alzheimer’s disease, leukemia, Parkinson’s disease, and cardiovascular disease.
There’s just one little problem here, and it’s called reality. Most of the research being done in these diseases use mesenchymal stem cells, which aren’t generally found in blood. So what stem cell type is LifeVault isolating? Try as I might, there is no info available on the site to answer that question. On their LinkedIn feed, they recently listed an Alzheimer’s study that involved adipose-tissue stem cells that were isolated and culture expanded. Given that none of that has anything to do at all with the blood being stored by LifeValut, it’s unclear why this study was posted. Another study listed on their feed used a unique protein that turns on myelin repair in the body, again having nothing to do with what LifeVault is doing.
I did get this response from the LiftVault CEO, Trevor Perry: “We are isolating specifics cells for induced pluripotent stem cell creation and hematopoietic stem cell progenitors.” That looks the same as this news story.
What Are iPS Cells?
Induced pluripotent stem cells (iPSC) are based on the idea that we can take adult stem cells and coax (or hammer) them, in the lab, back to their primitive stem cell state. The technology is new this past 5–10 years, and there have been significant concerns that these cells may not be all they’re cracked up to be. The biggest issue is that we have almost no clinical data that these “lab manufactured” stem cells will help any disease.
Forever Labs
Forever Labs takes a different approach, in that at least they’re using a stem cell source that has a significant number of stem cells and has early clinical data suggesting efficacy. They take a bone marrow sample, and their advertising is focused on storing your stem cells when you’re young. Their focus in on anti-aging. Whether this would work or not is anybody’s guess, but at least there is some research that shows that giving younger stem cells to an older person could possibly work to improve health.
One concern with this company came in a call I got from a colleague. Like all stem cell-storage plays I have ever seen, they always branch out. This colleague asked me if I thought that this was a good service to offer his patients. His intent was saving some of their bone marrow for a future treatment. For example, he would perform one bone marrow aspiration and then use some for the first treatment and save some for a future therapy. What I told him was that while at first blush this may sound like a smart idea, given that bone marrow stem cells are dose sensitive, for most clinical applications involving large joints and the spine, there wouldn’t be enough cells left over to store. In fact, the only “stem cell storage” concept that makes sense is if you’re growing the stem cells to larger numbers, but this isn’t legal in the U.S. This is only offered in our licensed Grand Cayman facility.
Decision Trees for Consumers
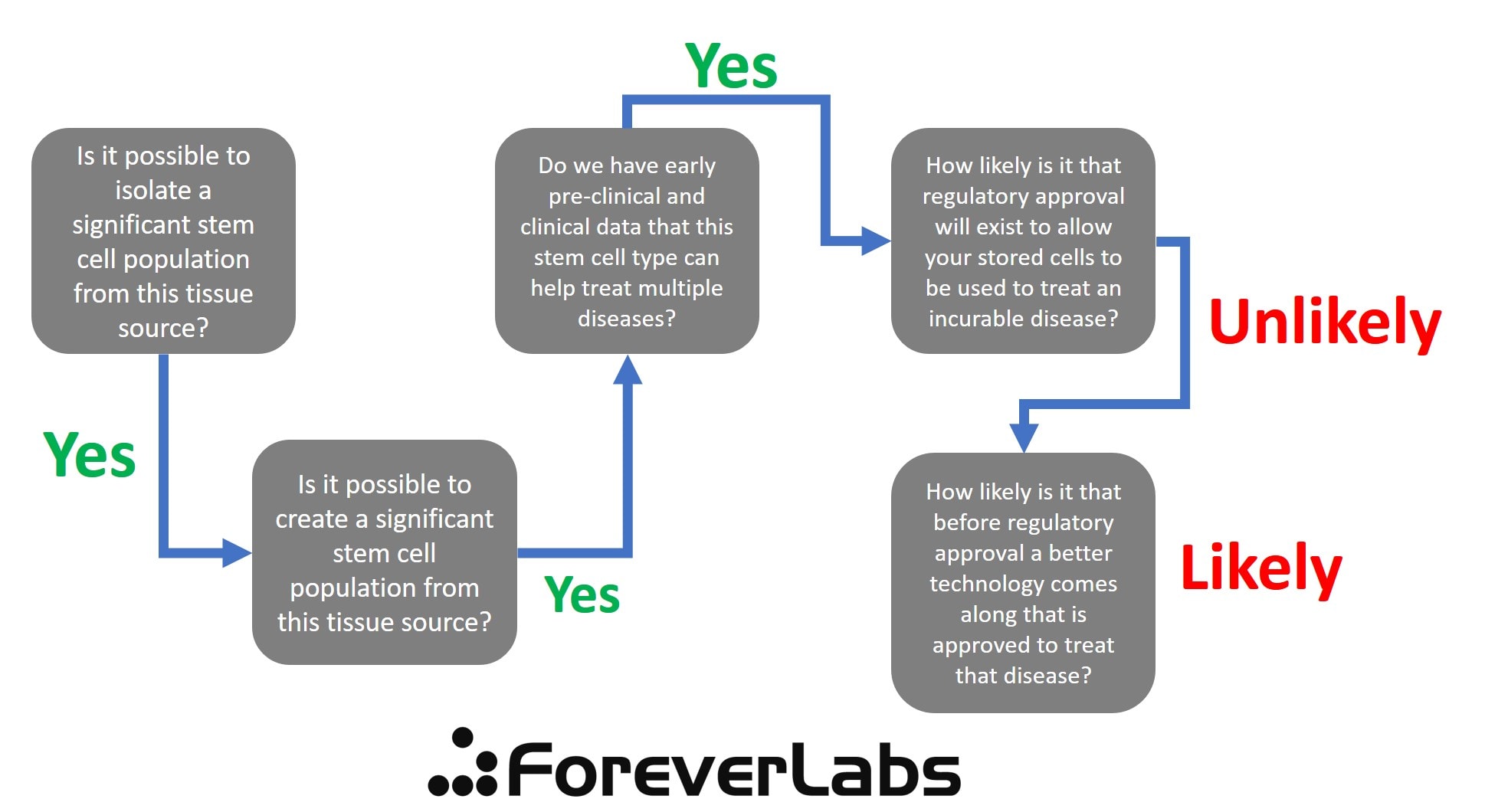
Is either company offering something that is likely to be useful to someone who is young now but as he or she ages acquires an awful and incurable disease? To get to that answer, I created a decision tree that asks the tough questions.
First, the biggest issue where all of these companies fail is that FDA approval for stem cells used to treat disease is VERY SPECIFIC. Based on the existing regulatory structure, there will NEVER be a blanket approval of a cell type (like mesenchymal stem cells or iPS cells) for therapy. That approval will tie a very specific lab process used to collect, isolate, and culture those cells to treat a specific disease. Hence, if you don’t use that lab process, there is no approval. This means that it’s VERY UNLIKELY that these stored cells will ever receive any FDA approval for use in any disease. To do that, these two companies would have to submit a several-hundred-million dollar IND to treat every specific disease. That’s VERY UNLIKELY to happen.
Below is the decision tree for LifeVault’s “GoodCell” product. As you can see, there are MANY issues, not the least of which are that while the company CEO replied that they will be creating iPS cells from this collection, and that may be possible, we have no early clinical data that iPS cells would be able to cure any of the diseases listed. It’s also a concern whether iPS cells could be created after a sample of blood is shipped across the country, isolated, stored for 20 years, and then thawed. While it’s likely you could make that work, it’s not a sure thing (which I why I have “Maybe” at that step). However, even bigger issues happen on the regulatory side. Meaning, if iPS cells could be approved for some future clinical use of a specific disease, that approval will be tied to a very specific collection and lab process and company performing that process. If that company is not LifeVault, these cells may not be able to be legally used for that purpose.
Forever Labs has an easier decision tree, but again gets hung up on the regulatory side. So while there are MSCs in bone marrow and there is good early-stage research showing these may help treat multiple disease types, the same issues happen on the regulatory side. Any MSC approval will be tied to a specific company and specific process of isolating and growing cells.
Won’t These Companies Be Able to Send My Cells to an FDA-Approved Company That Can Isolate, Create, or Grow Them?
While this could be theoretically possible, this has not happened in any FDA cellular product approval to date. The process of isolating and storing the cells also goes through the FDA approval process and is as integral as creating or growing cells. Hence, it’s VERY UNLIKELY that any company that eventually gets an FDA approval to use stem cells to treat disease would be able to use stored cells from either company.
The upshot? Nobody likes to buy an expensive insurance plan that may never pay off. While it’s possible that either company’s product may work to someday cure disease, that seems very, very unlikely sitting here in the early part of the 21st century. Hence, please think before you leap.
___________________________________________
Note, I did get a detailed response from one of LifeVault’s founders, Harvard professor David Scadden, that I thought in all fairness I felt I should publish:
“Thank you for your note, Chris.
Since that seemed reasonable, the idea was to be sure that there really were things coming down the pike that indicated iPS cells would be useful for people. iPS cells as you know, have provided pre-clinical sources of cells therapeutic in many models of disease either as the therapy or as the basis for defining drug therapies. Several leading scientists who have a record of testing that concept and moving it to a clinical setting were contacted and engaged. The idea was that they might give information about what was currently being investigated and also what was on the horizon based on the best science available. In addition, an expert who currently runs cell processing centers and is an expert in stem cell therapies was included for that critical perspective.
It was also proposed that, if cells were to be preserved, that it might be good to also save plasma since new tests were being developed that used protein or DNA in plasma to determine disease risk factors. The experience with blood tests for risk factors in general is that the tests are often best interpreted in the context of a baseline. Having a stored plasma sample could then help with providing such a baseline.
Finally, it was felt that having some genetic analysis done at the time of collection could potentially provide some useful information for people in more real-time. LifeVaultBio then engaged a leading human geneticist (who is skeptical about overuse of genetic testing) to guide what genetic tests might be useful.
All this was put together to provide a potential resource for those most proactive about having access to health technologies and most optimistic about what research in the stem cell field might yield. It was never to be promising a current therapy. It was to help give some information now and access to blood cells and plasma cells for the individual later should the technology advance in a way that some scientists in the field thought it might. The plan was also to give written information to those interested on a regular basis informing them of advances in the regenerative medicine field. If the marketers went beyond this, it is against what was the agreed upon plan and violates what is at the core of what all of us on the science side hold dear. I have asked that the name of all the scientific advisors be removed from any and all materials pending management sorting this out and to pull the website. Whether I am associated with any of this going forward is to be determined, but I assure you, the goal is to provide real value to people based on the best science.
I hope this clarifies at least the background for how this got started. I would be happy to speak with you directly, if you would find that helpful.”

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.