Congressional Hearing: The FDA is the Cause of the Drug Shortages
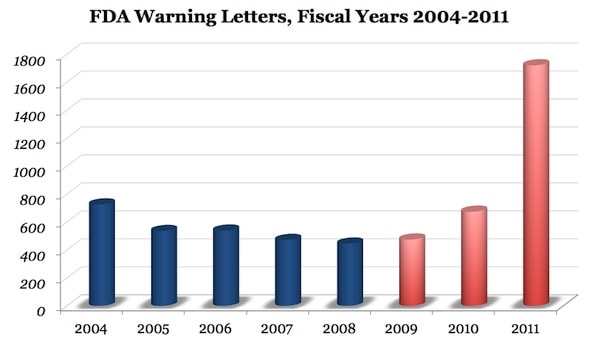
Is the FDA causing drug shortages? We’ve been seeing the effects of the drug shortages in the clinic. As an example, we were paying about $1 a bottle for local anesthetic, now when we can find it-that same bottle is often $4 a bottle. This is really been impacting patient care, as the surgery center we work out of can’t get certain drugs. More significantly, cancer patients have been dramatically impacted, as cheap generic drugs are being impacted the most. What’s interesting is that about 6 months ago I figured these shortages were from an ascendant hyper-regulatory FDA. Well, a congressional hearing just confirmed it, blaming the FDA for these shortages. What’s happening is that our “new FDA” is overly focused on reducing what’s been called type 1 regultory error (what happens if a danagerous drug is let on the market). While that’s admirable, it’s also fool hardy to only focus there-as the more you persue a singular policy of reducing type 1 error, the more you increase type 2 error (what happens when you keep good drugs from the market). Since the number of people harmed by type 2 error is massive (by one estimate the delay of approval of one cancer drug by one year costs 82,000 lives a year), it’s unknown why our FDA is only focused on reducing type 1 error. Forbes last week pointed out the huge rise in Warning Letters issued by our new FDA (see above) and how that has shut down many Generic drug makers. The issue is that these factories were shut down not because there was an actual problem, but because of theoretical risks due to cGMP violations. The FDA employees that issued the letters did not take into account the consequences of their actions (that the entire factory would have to shut down). Many of these manufacturers may or may not be able to reopen production lines for these cheap medications due to their increased regulatory costs (some have had to build new facilities). Interesting-this means less generics? What’s also problematic is that for many of these drugs, there are no brand name alternatives anymore (like lidocaine). The upshot? We have a hyper-regulatory FDA that’s focused on reducing one type of regulatory error and by being only focused in one direction, they place the public’s health at risk. The solution? We need a FDA that’s as concerned with type 1 error as type 2 and seeks to balance these so that the public is protected from bad drugs as much as it’s protected from delays in promising therapies or shortages of generic drugs.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.