UC Expanded Stem Cells in Arizona? How the Bernal Decision has Caused the Stem Cell Wild West to Spin Out of Control

In late August of 2022, a much anticipated and COVID-delayed legal decision was handed down on using stromal vascular fraction and FDA approval. IMHO, that local decision has now had wide-reaching, national impacts on the stem cell wild west beyond what any credible attorney would tell you is the law. Let’s dig into this topic through an Arizona naturopath that uses culture-expanded umbilical cord MSCs.
The Bernal Decision
What is the Bernal decision? This is the USA v. California Stem Cell Treatment Center case that was decided last year. Let’s dig in.
A handful of providers in the United States began using a fat stem cell product around 2012. That product was made by taking adipose tissue using liposuction and then digesting it with an enzyme. The final digested product was then centrifuged, much like bone marrow, to concentrate the stem cell fraction. The final product was called “Stromal Vascular Fraction” (SVF) and contained mostly non-stem cells, with a small percentage of the remaining cells qualifying as mesenchymal stem cells.
As the number of providers offering SVF rapidly increased throughout the twenty-teens, the FDA drew a line in the sand and took the position that using an enzyme to digest the fat created a drug product. This forced legitimate players to seek FDA drug approval to create devices that made SVF. However, there were two large holdouts. One was a Florida company called US Stem Cell that challenged the ruling and went down in flames in court, with the FDA easily winning its case against them. The second was Cell Surgical Network, which challenged the position in California. The judge, in that case, was Jesus Bernal, who did not drink the FDA Kool-aid and ruled against the agency, stating that SVF wasn’t a drug. The FDA has now appealed the case to the 9th circuit.
Will the FDA win its appeal? Betting against the government when defining what it can and can’t regulate has a history of heavily favoring the home team. Meaning that not only do the feds win the vast majority of these cases, but they tend to begin these cases with the ball placed on the opponent’s 10-yard line and spotted a dozen points. However, just like the Bernal decision, stranger things have happened.
The Stem Cell Wild West
I would love to say that using orthobiologics is rational and follows a slow and measured adoption curve as more and more evidence is created. That providers are careful about what they claim and only offer to treat problems that can reliably be treated with minimal risk to the patient. Regrettably, there are only a small handful of medical providers in that category and for every one of those, there are a considerable number of usually alternative healthcare clinics ignoring the science and offering magic. I call that large section of this field “The Stem Cell Wild West.”
The Impacts of the Bernal Decision on the Stem Cell Wild West
The Bernal decision was in the 9th circuit in California. This is a district court decision that only applies to Los Angeles. Not even the other district courts in the 9th Circuit in California are bound by this decision. It certainly doesn’t legally apply to the rest of the nation. Despite these severe limitations, IMHO, we’re now seeing US providers using it as one of the reasons to push the envelope on what’s legal.
For example, based on existing FDA regulations and case law, we know that you can’t use autologous culture-expanded stem cells in the US without FDA drug approval. This refers to the patient’s cells grown in culture to expand their number. That doubly applies to allogeneic cells like those obtained from an umbilical cord. However, while perusing Facebook, I came across an Arizona naturopath using culture-expanded umbilical cord MSCs (Mesenchymal Stem Cells). That caught my interest.
For example, here’s the naturopath named “Dr.” Travis Whitney. I spent some time trying to get him to outline a legal theory as to how he can use these cells despite the FDA’s position that they constitute an unapproved drug product:
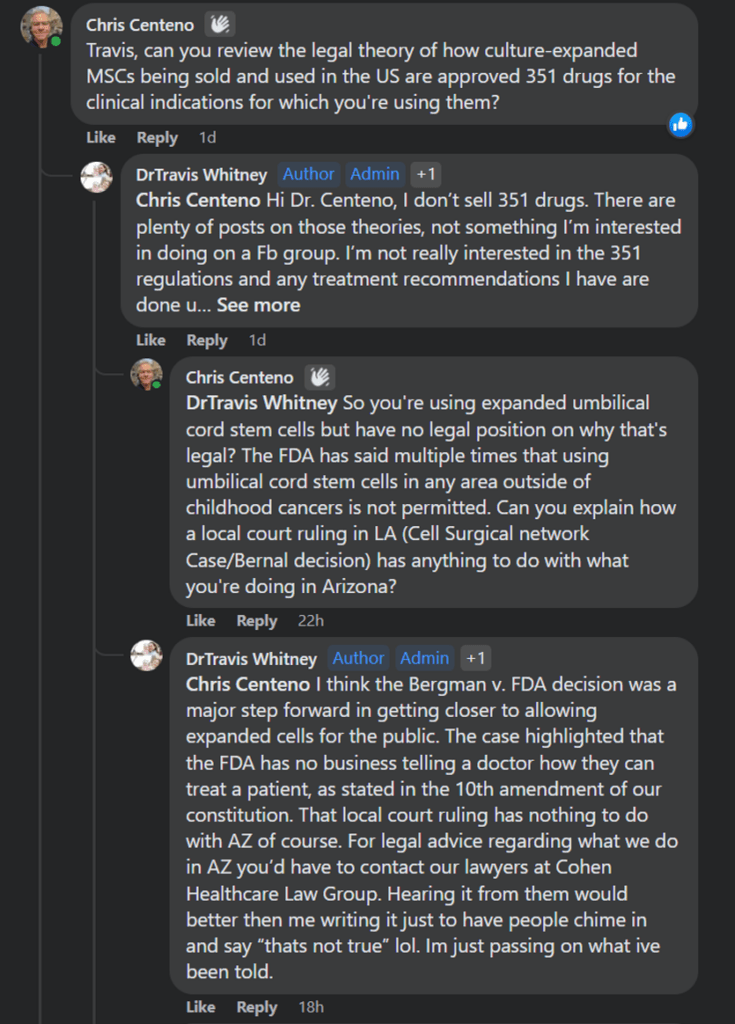
Dr. Travis Whitney believes that, partly because of the Bernal decision (what he erroneously calls Bergam v FDA which is a misspelling of Mark Berman’s name, one of the defendants in the FDA lawsuit), he can use culture-expanded umbilical cord MSCs. The other claim is that the 10th Amendment to the US Constitution gives him the right to use these stem cells in Arizona.
What does the 10th Amendment have to do with physician practice of medicine rights? This is the federalism portion of that document. The argument would be that since the states regulate the practice of medicine, the federal government can’t tell a physician how to practice medicine. However, “Dr.” Travis is not a physician and never went to medical school, internship, nor residency. He’s a naturopath. Hence, the argument would be that the state of Arizona’s statutes or naturopathic board rules gives him these rights, which supersede the federal regulation of umbilical cord stem cells as drugs.
I asked “Dr.” Travis for his legal opinion from “Cohen Healthcare Law Group” that he referenced above, but I never got anything approaching that document. If I ever get that, I will post it here.
What “Dr.” Travis Claims
On his website, we see this claim made by “Dr.” Travis:
“Innate Healthcare Institute is a unique entity in the field of stem cell therapy for joint pain as our laws and medical board in the state of Arizona allow for the administration of higher doses of umbilical cord mesenchymal stem cells typically only found in over-seas clinics. The course of treatment is therefore between the doctor and the patient.”
So where are these laws and medical board rules? Let’s dig in there.
Arizona Right to Try Law
Is there a specific law in Arizona that gives “Dr.” Travis the ability to use culture-expanded umbilical cord stem cells? Nothing that I can find has passed the legislature. A bill similar to that was attempted in 2019 but was killed in committee.
In 2014 Arizona passed a “Right to Try” legislation for patients with terminal illnesses. This was for drugs that have passed phase I of an FDA trial. That law was expanded to include individualized genetic drugs in 2022, IMHO, none of this applies to using culture-expanded MSCs to treat knee arthritis or back pain by an Arizona naturopath.
Arizona Naturopathic Practice Act
What does the Arizona Naturopathic Practice Act tell us? First, after reviewing the entire document, nothing is described in the practice act about naturopaths performing spine or injection procedures. This is important, as “Dr.” Travis claims to perform these procedures.
I’ve blogged on this issue before of naturopaths performing procedures like bone marrow aspiration, spinal injection, and liposuction. I couldn’t find evidence that the naturopathic practice acts around the country would authorize a non-physician to perform these procedures. I asked my colleagues about this issue, and they all came together to create a document denouncing this practice. That’s because the consensus was that naturopaths did not have the proper training to perform these procedures safely.
To that end, it’s important to review Britt Hermes’ website. Britt is a former naturopath who is now a Ph.D. student and has written about the problems with the profession. Her personal experience is that after attending what is arguably the “Harvard” of Naturopathic schools (Bastyr in Washington), her education was woefully inadequate to care for sick patients safely.
Second, is there a naturopathic medical board rule on using stem cells? I went to www.nd.az.gov and clicked on the Board Rules. I searched the document and came up blank on “stem cells.” The board describes that naturopaths can use natural substances, which are defined as follows:
“Natural substance: means a homeopathic, botanical, nutritional or other supplement that does not require a prescription pursuant to federal law before it is prescribed, dispensed or otherwise furnished to a patient and that is prescribed by a physician who is licensed pursuant to this chapter to enhance health, prevent disease or treat a medical condition diagnosed by the physician.”
IMHO, culture-expanded umbilical cord MSCs wouldn’t fit under this definition. I tried calling the board several times but could never connect to a human. I also asked “Dr.” Travis to explain his statement with some references I could look up, but I never heard anything. I will update this blog if he replies.
The 10th Amendment Argument
As mentioned above, the idea behind federalism is that there are things that the federal government controls, and there are things that the states controls. As you can see, no legislative or board support exists that “Dr.” Travis can use culture-expanded MSCs as a naturopath in Arizona. Despite this, there would still be an argument that his state naturopathic board gives him these rights (hard to make given the practice act I read), and hence those supersede federal drug laws. For example, Marijuana is illegal federally, but several states have expressly stated that it’s legal medicinally and/or recreationally.
Does the 10th Amendment argument, in this case, hold water? That depends on whether the federal government has decided not to regulate or prosecute. That’s what’s happening with the DEA in the case of marijuana. How about umbilical cord stem cells? The FDA has made it clear in numerous untitled and Warning Letters that it regulates umbilical cord stem cells as a drug that require FDA approval and regulation (letter 1, letter 2, letter 3, letter 4, letter 5, and letter 6). Are the culture-expanded umbilical cord stem cells being used by “Dr.” Travis FDA-approved? Nope.
On that last note, it should be noted that the feds also have a history of prosecuting individuals in Arizona for selling umbilical cord stem cells, but this wasn’t FDA, but the FBI.
Is Using Culture Expanded UC MSCs Safe?
Almost all of the published work on using umbilical cord MSCs for orthopedic problems has been published in Asia. The problem with using these studies to claim that the umbilical cord MSCs you’re using are the same as the ones described in these studies is that the culture process can change the cells. Let’s explore that more below.
Small changes in culture conditions make significant changes in the cells. Here’s a short list of all of those complex variables:
- How are the cells isolated before culture?
- What are the specific requirements for umbilical cords, and how are those validated?
- Are the cells early or late passage?
- What culture media is used?
- Are they cultured using hypoxic or normoxic conditions?
- How are the cells plated, and at what density?
- On what type of substrate are they plated?
- How are the cells produced by this process assayed so we know that batch A is the same as batch B? How was that assay validated?
- Are they grown in a cGLP, cGTP, or cGMP lab?
- What are the cell release criteria?
- How about shipping criteria?
- How were the release and shipping criteria validated?
- What are the thaw criteria?
- Was only a simple live/dead stain used for post-thaw viability or an apoptosis stain?
- At what exact rate are the cells thawed?
I have published dozens of peer-reviewed research papers in this space, and several book chapters. Based on that experience, the abovementioned variables are often not found in peer-reviewed research papers on umbilical cord stem cells. Meaning UC-MSC line A found in a random paper produced in Asia is likely not like the culture-expanded cells that any individual could purchase here in the US.
I asked “Dr.” Travis the questions above about the cells he’s using and have yet to hear any answer. If I get that information, I will update this blog.
The upshot? I know I say this a lot, but you can’t make this stuff up. I was floored to see a naturopath in Arizona using culture-expanded umbilical cord MSCs. Ultimately, I can only conclude that the Bernal decision has impacted this space and that what I’m seeing is just the tip of a massive, but partly submerged iceberg. In other words, this is likely happening out there much more than we know.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
