Growth Factor Content of Amniotic and Umbilical Cord Products

Credit: Shutterstock
We have been testing various umbilical cord and amniotic products for live stem cells, but we also sent these samples for independent growth factor analysis and compared those metrics to a weak platelet mixture. What did we find? Read on…
Do Umbilical Cord Products Have Live Stem Cells?
We’ve tested many birth-tissue products derived from umbilical cords and have yet to find any with mesenchymal stem cells. This is despite the claims of the manufacturers about these products. These past few weeks, we tested two more: LiveyonPURE and StemVive from Utah Cord Bank.
Liveyon PURE is a cord-blood product that claims to have live MSCs, and StemVive is derived from the Wharton’s jelly part of the umbilical cord and also claims the same thing. Our tests of these products demonstrated no living mesenchymal stem cells (MSCs) in either Liveyon PURE or StemVive that survived to be able to culture; whereas, middle-aged bone marrow produced many live MSCs.
However, many of these companies also claim that their products have very high levels of growth factors (GFs). In fact, that comparison is made to convince doctors that these young “cell” products (that are really dead-cell products) have much higher GF levels than the patient’s own PRP. Hence, we also sent these samples out to an independent lab (RayBiotech) that tested their GF and cytokine levels against a weak PRP. What did they find?
What Are Growth Factors?
Think of GFs like espresso shots for cells. They can enhance a cell’s activity just as a triple-shot espresso can enhance yours. Hence, the claim that these products have high GF levels may help their ability to act as substances that can heal tissue despite their lack of living MSCs.
The 30,000-Foot Comparison
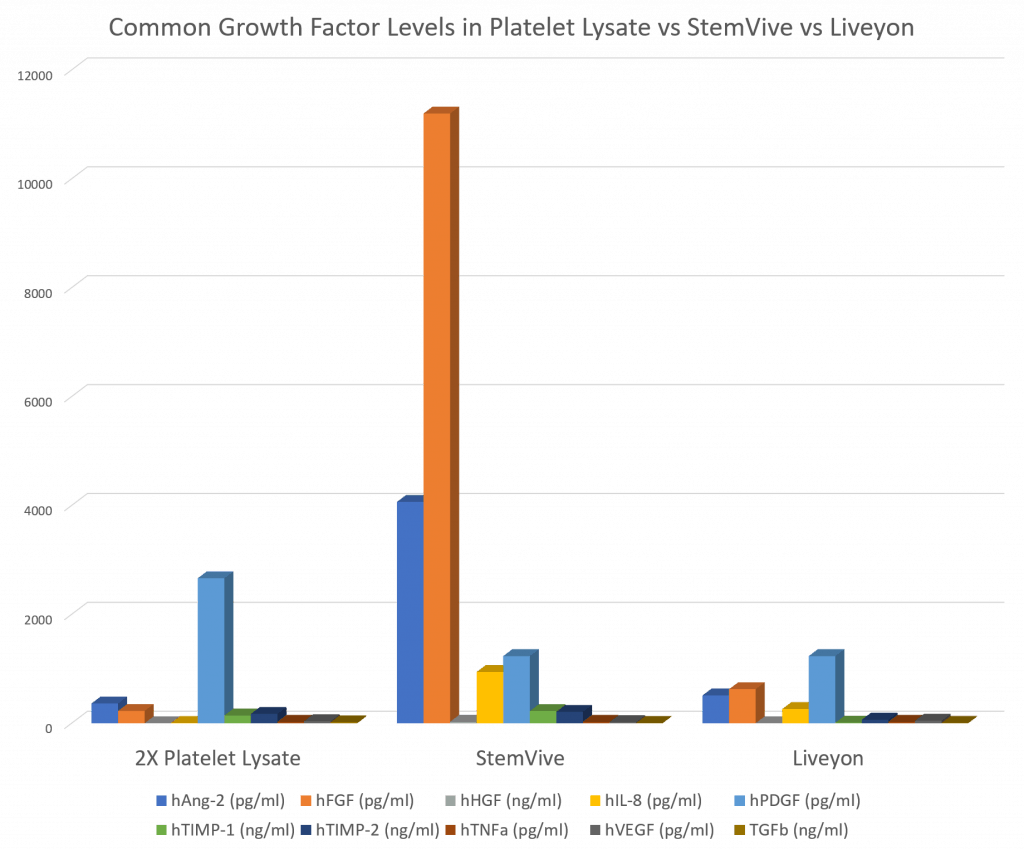
The graph above is an overview of the GF levels in a random weakly concentrated platelet sample, StemVive, and Liveyon PURE. So that this all makes sense, I’ll compare each GF one by one along with first explaining what that GF does. We’ll also look at some good- and bad-actor cytokines that RayBio also measured.
Before we get started, it’s worth taking a minute to understand what we used as the control sample. For that, we sent a random platelet sample from one of our patients and extracted the growth factors from that sample, which only contained two-times concentration. Hence, these growth factor levels are roughly equivalent to those found in a very weak platelet-rich plasma.
Platelet-rich plasma (PRP) is a cocktail made from the patient’s own blood that contains lots of healing growth factors. Given that our lowest concentration PRP that we would commonly use in most patients is 7 times concentrated, and that goes all the way to 14–20 times, suffice it to say that these growth factor levels that you see here are a fraction of those found in our most-common Regenexx PRP treatments.
Hence, realize that if we want to increase the GF concentrations in any of these PRP preps, all we have to do is to increase the concentration of that PRP. Meaning, if want to double the growth factors found in this weak 2X prep, we just make a 4X. If we want to double that again, we can take more blood and make an 8X and so on.
TGF-beta
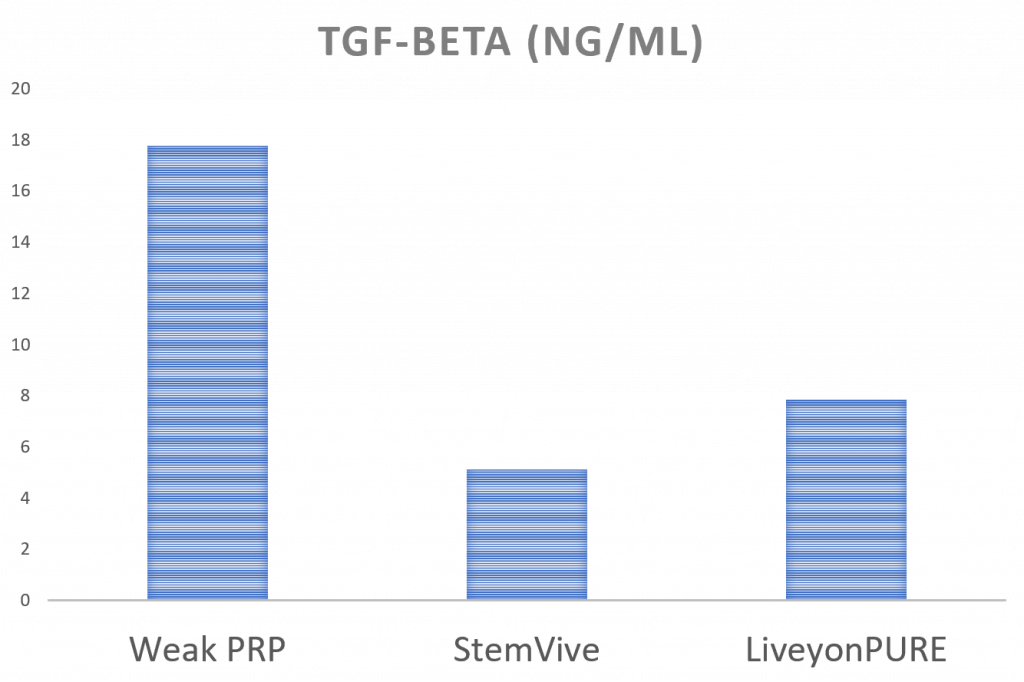
TGF-beta is an all-around growth factor that’s good at helping many cell types grow. Hence, it’s a great GF to have in your therapy. Here a weak PRP has much more TGF-beta than either umbilical cord product.
VEGF
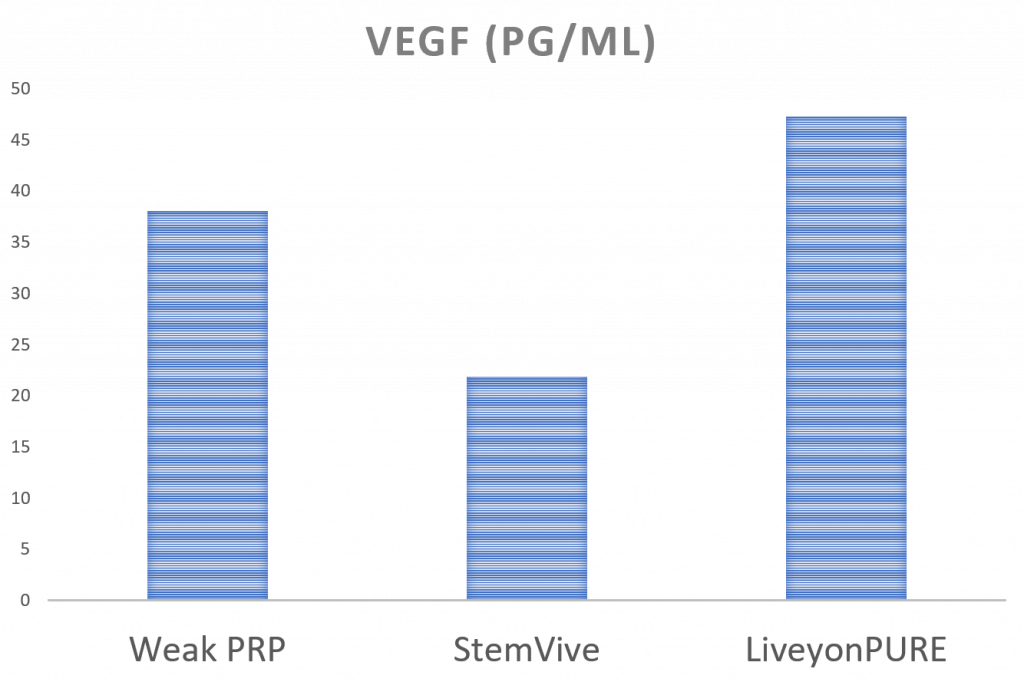
VEGF is a growth factor that helps new blood vessels grow. Since new blood vessels are needed in the healing process, this is a critical GF. Here a weak PRP has about the same amount of VEGF as Liveyon PURE, and the StemVive product has less. As discussed above, since the most commonly used concentration by Regenexx is 7X–14X, our typical PRP would contain much more VEGF than either umbilical cord product.
A Good-Actor Cytokine: Meet the TIMPs
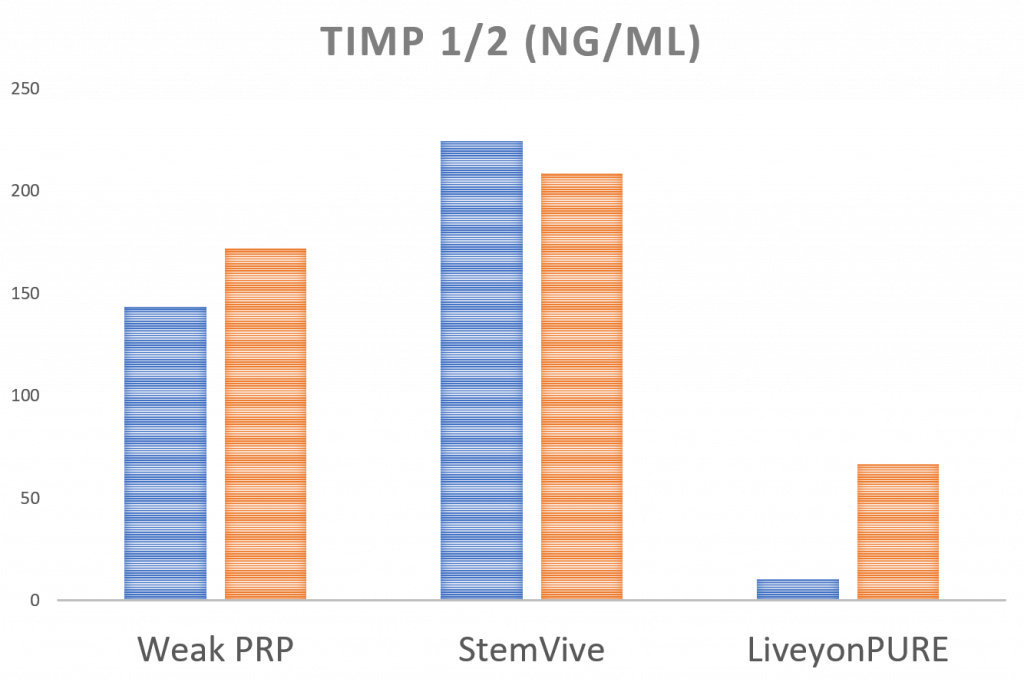
The TIMPs are anti-breakdown cytokines that may help protect joints against arthritis. Here the StemVive has higher levels, similar to a weak PRP. The Liveyon PURE has low TIMP levels. Again, a 7X–14X PRP would have much higher TIMP levels than either umbilical cord product.
Meet the IL-8 Bad Guy
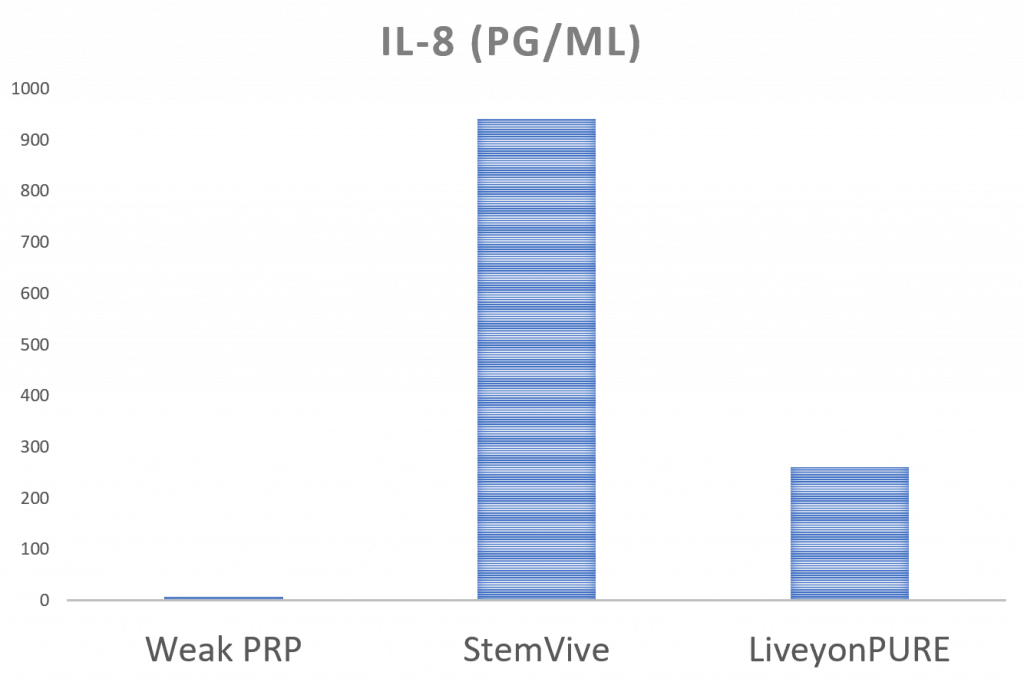
IL-8 is an inflammatory cytokine that has a number of functions, including causing inflammation by attracting white blood cells to the area. More is bad; less is good. Here, both umbilical cord products have awfully high levels of this stuff compared to a weak PRP. We also tested another inflammatory cytokine called TNF-alpha, and none of these three products had much.
How high are these levels? Very high! For example, serum blood levels of IL-8 in knee arthritis patients are generally lower than 10 pg/ml. Liveyon PURE’s levels are almost 300, and StemVive is more than 900. Ouch!
bFGF
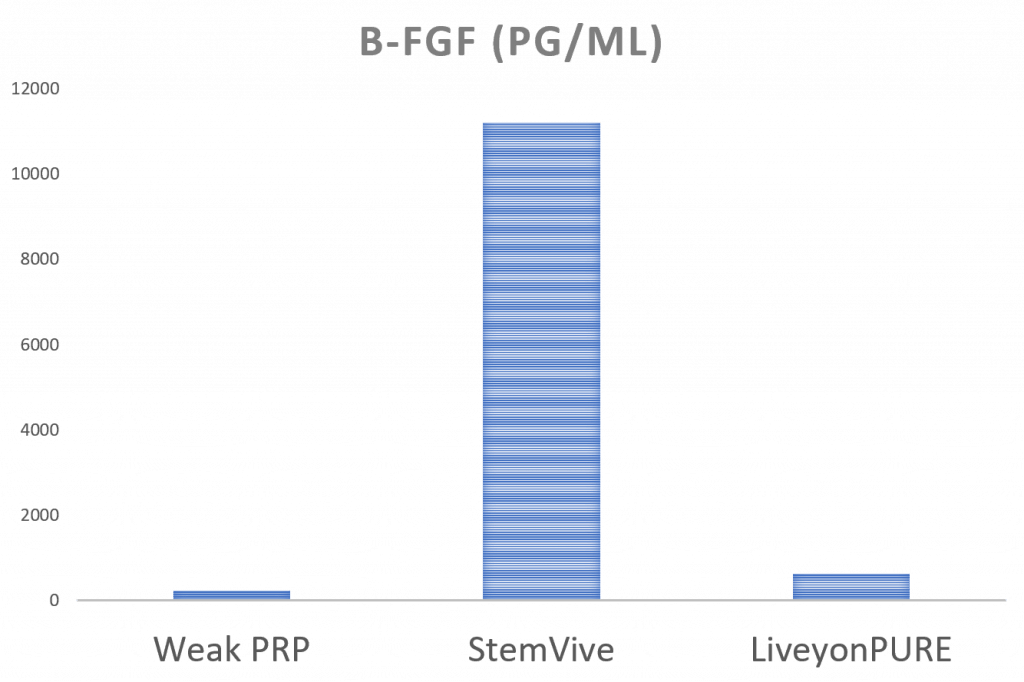
If there’s a bright spot in the GF and cytokine levels of these umbilical cord products, it’s the FGF levels. This is a growth factor that promotes the growth of tendon cells. So this may be why we see some medical providers noting nice results with birth tissues in applications like rotator cuff tears. While we also have reported home run results with treating partial rotator cuff tears with PRP and complete nonretracted tears with bone marrow concentrate, the FGF level in the StemVive product is very high.
What else can we compare to StemVive? The Interventional Orthopedics Foundation (IOF) tested two other birth tissue products, one that was made from amniotic fluid (BioD Restore) and another from the chorion membrane (Ovation Matrix):
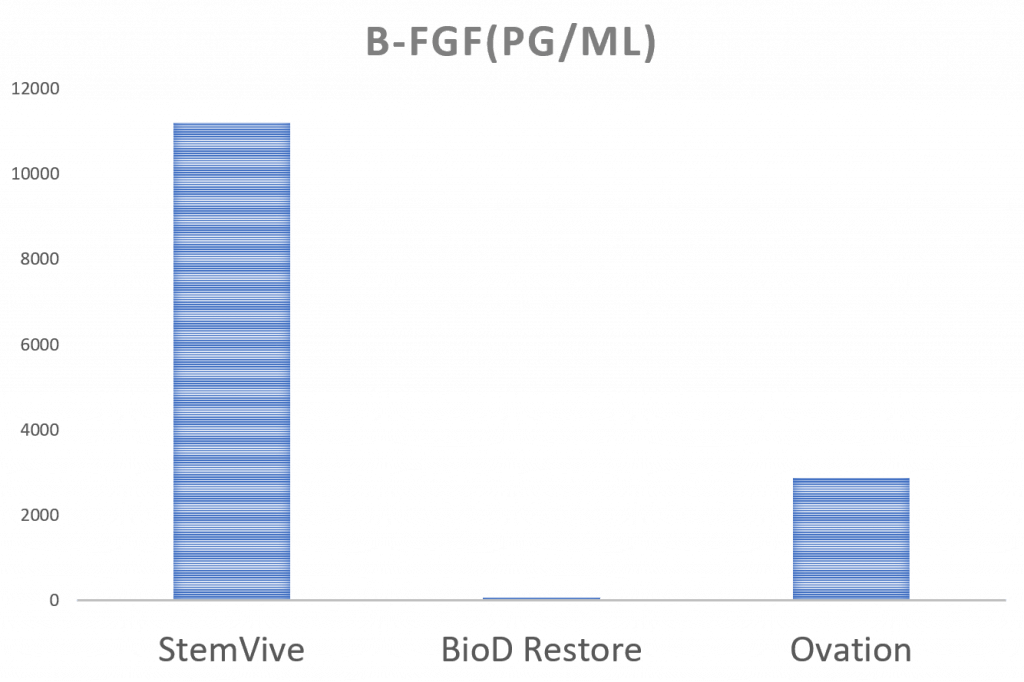
So StemVive still does well in this comparison. The big question would be whether these high FGF levels can be replicated across lots of StemVive. We have a second sample that we’ll test, and we will eventually report that data as well
The upshot? These umbilical products have no MSCs. For most of the common orthopedic tissue-healing growth factors, these two products have low levels compared to the types of highly concentrated PRP we use. They also have concerning levels of IL-8 inflammatory cytokine. Having said that, the FGF level found in this StemVive sample is impressive. How that translates clinically is unknown.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
