How Can Regenexx Satisfy the Requirements to Add Orthobiologics to a Health Plan?
Regenexx is the only orthobiologic provider on the planet that has been able to document the reduction of orthopedic costs by adding its services to a company’s health plan. How is that possible? Why has only Regenexx been able to do this? Let’s dig in.
What are Orthobiologics?
Orthobiologics are substances that can help damaged musculoskeletal tissues heal or can mitigate degeneration. These include many categories, but PRP (Platelet-rich Plasma) and BMC (Bone Marrow Concentrate) are the two most popular orthobiologics in use today. Watch my short lecture below to learn more:
What is Interventional Orthobiologics?
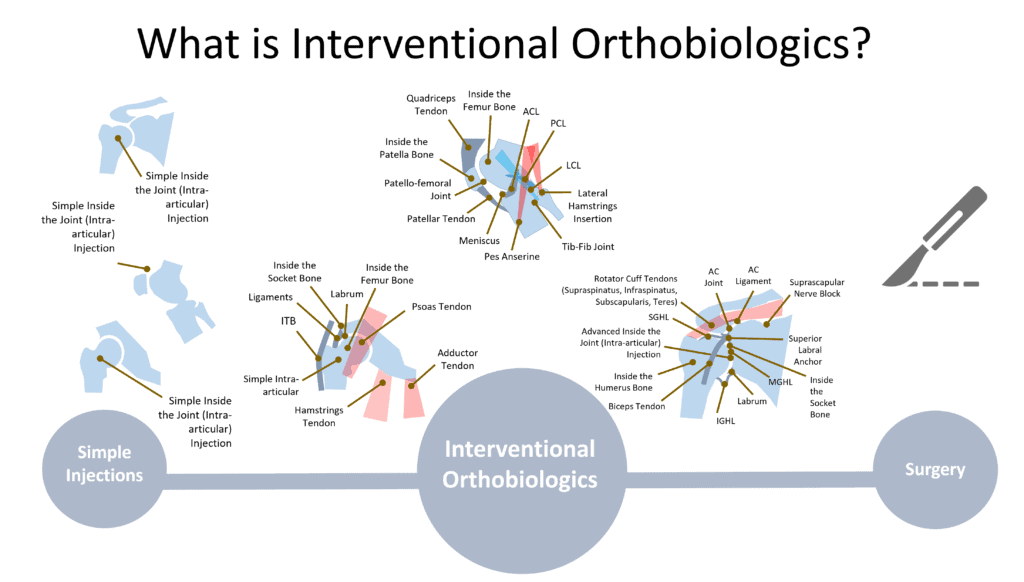
Interventional Orthobiologics, also called “Interventional Orthopedics” (IO), is the precise image-guided injection of orthobiologics to aid in healing or the mitigation of degeneration in orthopedic injuries and diseases. This discipline sits between conservative care like physical therapy and invasive care like orthopedic surgery. For example, a patient who may usually require ACL reconstruction to treat a damaged ACL can instead be offered a precise x-ray guided injection of bone marrow concentrate to help heal the injured ligament.
To learn more, watch my video below:
Is There Research Showing that IO Works?
The best evidence base to date for the efficacy of orthobiologics are in the fields of PRP and BMC. For example, we have a huge and significantly expanding research base of high-level RCTs supporting that PRP is effective for various applications including knee osteoarthritis, shoulder tendinopathy, epicondylitis, tendon injuries, and carpal tunnel syndrome (5-54). Bone marrow concentrate has an evolving evidence base strongest in treating moderate to severe knee osteoarthritis, with some evidence of efficacy in tendon injuries (1-4).
Why Add IO to Health Plans? What’s In It For the Company?
If you’re an employer, why add IO to your benefits package? Cost savings, productivity, and employee retention. On the savings side, orthopedic surgery is one of the largest drivers of health care costs. Based on actual case studies, by our providers offering employees lower-cost IO procedures instead of surgery, we can save 40-70%. These precise injection-based procedures are also MUCH less invasive, which means much less time off work and downtime. Finally, keeping employees during the great resignation is tough. Hence, adding unique benefits like orthobiologics is one way to show employees that management is working hard to keep them.
Standardizing Everything is the First Hurdle to Adding an Investigational Treatment to a Healthplan
While the published research behind IO is strong in many areas and building in others, most physicians would still consider IO investigational. If that’s the case, how is it possible that Regenexx has been added to the self-funded health plans of more than 500 companies? Before we get there, let’s go over what Regenexx as a company has done to make it VERY different from anything else out there in the field of orthobiologics.
While orthobiologics are transformative for musculoskeletal care, adding this benefit carte blanche without controls would likely cause costs to go up as orthopedic offices begin to add these procedures to existing surgeries instead of replacing those procedures. In addition, there is no standardization in this field, so what gets offered to patients would be all over the proverbial map. That would include physicians offering patients orthobiologics in clinical scenarios where they are clearly ineffective. For example, our research shows that offering a bone marrow concentrate injection to a patient with severe hip osteoarthritis is a waste of time as the response rate is very low. How does Regenexx overcome these hurdles? Education and standardization. Here’s a short list:
- A National Network of Physician Super Specialists—We now have about 75 geographically dispersed US sites and will add approximately 15-20 this year. Given that our physician network began a decade ago, we could have added many more sites, but we have strict requirements for who can be added based on physician specialty and experience in image-guided procedures. Meaning, that most physicians that would be interested are not qualified to be added to our network. Why? When your goal is saving money by substituting IO for more expensive and invasive surgery, you need to be able to deliver on that promise by keeping physician quality high.
- Physician Training—How do you keep hundreds of physicians on the same page? That requires extensive training requirements. While in older fields of medicine, training requirements would be handled by physician board certification, in the newer field of IO, there isn’t a board yet to impose minimum training standards. Hence, we spun off a training non-profit called the Interventional Orthobiologics Foundation (IOF), which has now taken on a life of its own. Our doctors must take and pass this course-work. We also have Regenexx-specific training requirements that are layered on top of IOF.
- Orthobiologic QA—Even if you could establish a national network of physicians and then make sure their training was similar, the problem would be that each practice would inject different orthobiologics. For example, what constitutes PRP? How concentrated should the platelets be? How is that dosed? Should it be leukocyte poor or rich? Which type gets injected for which conditions? If we take a dozen practices experienced in injecting PRP, most would have wildly different answers to these questions. Meaning that there is no “Starbucks” out there, making the PRP “Latte” the same way every time. At Regenexx, we standardize all of this so that each clinic can use a consistent product in the same clinical scenario to produce consistent results that can be measured.
- Outcomes Tracking—Even if you could create a national network, standardize physician training, and have everyone inject the same stuff, in the same way, every time, you would then have the problem of measuring outcomes. At Regenexx, we have the world’s largest and oldest patient outcome and complications registry that’s been around for almost two decades. Why do this? One reason is to identify clinical scenarios where orthobiologics don’t work and make sure the entire network knows to avoid using IO in those cases. Can’t any group just bolt a registry onto a medical network? While a few companies can now act as an overlay to track outcomes, this creates new challenges. For example, getting individual practices to enter new consented patients into a registry is like herding cats. Why? This requires significant changes to the workflow of the medical practice. At Regenexx, we’ve had two decades to master tracking outcomes.
- Utilization Review—As discussed above, how do you keep providers from using orthobiologics in cases where they don’t work? Or substituting a more expensive IO procedure for a much cheaper item like a few visits of physical therapy? At Regenexx, we’ve developed the world’s first orthobiologics utilization review system where providers can tell us what they’re doing and why and get instant approval to move forward or get flagged for medical director review.
- Publications—Finally, while no other group of physicians controls all of these variables, there’s still one key component: peer review. In other words, is that group taking those tracked outcomes and routinely publishing them in the peer-reviewed literature? Only Regenexx has done this to date (all Regenexx publications at this link). Regenexx has published large datasets as well as several randomized controlled trials. We have also investigated key issues from the in-vitro side of the equation, like what’s the right PRP dose based on age? (55)
The Mechanics of Adding an Investigational Treatment to a Healthplan
While all the above keeps everyone on the same page and allows us to inject the same stuff in the same way and track outcomes, how does an investigational therapy get added? Let’s review that below.
The type of health plans we’re discussing here are self-funded. What doe that mean? Most medium-sized to large corporations buy health insurance very differently than the public. They have enough money in the bank to write the checks to providers directly. All they lack is a provider network and claims handling, so they contract with a Third Party Administrator (TPA) for those services.
Every TPA has a policy regarding experimental and investigational treatments (E and I). As already reviewed, orthobiologics to treat orthopedic conditions are considered experimental and investigational. We agree with that conclusion as adding all providers in the “stem cell wild west” would be a disaster for health plans. So how does a self-funded insurer and its TPA decide when a better controlled new technology like Regenexx meets the requirements for inclusion in a health plan?
To test Regenexx against an E and I standard, let’s look at a health plan policy from Blue Cross Blue Shield of Massachusetts. Most BCBS plans use the same policy, which has been in place for at least a couple of decades. The policy lists five guidelines that you must meet for BCBS to cover new technology. There are 5 key requirements:
- Regulatory
- Evidence
- Improving Net Health Outcomes
- At Least Equivalent to the Established Treatments
- Technology Available Outside of a Clinical Trial
Regulatory
In this category, if an FDA approval is required for the investigational care, is one available? There is no issue if an FDA approval is not required. Here, PRP and BMC are both regulated as medical procedures that fall under the 21 CFR 1271.15(b) exception allowing physicians who create minimally manipulated biologic products from the same patient and back into that patient (autologous). Hence, both of these products require no “FDA Approval,” and the regulation falls under the same category as a knee arthroscopy or a back surgery, which are regulated by the state medical board. So while the tools needed to perform the procedure could require device approval (like a knee arthroscope or scalpel), all of the tools used by Regenexx are 510K cleared or exempt because they are in the general laboratory use category.
Evidence
A specific level of evidence is required to meet the BCBS investigational standard. Here the evidence should be published in peer-reviewed journals, be of good quality, and show a positive impact on health outcomes. Given that dozens of Randomized Controlled Trials (RCTs) have been published that show that PRP can positively impact pain and function using validated questionnaires, PRP meets this standard (5-54). BMC also meets that standard when treating knee osteoarthritis (1-3). Finally, professional organizations have taken notice of this BMC data and published positive position statements (63).
Improving Net Health Outcomes
Here the BCBS guidelines discuss that the positive impact of the technology on health outcomes should exceed the negative impact. For example, we know that lumbar fusion has a questionable positive health outcomes impact and has serious negative impacts, including complications such as infection, revision, and adjacent segment disease (56). For interventional care, the complications are much less than those that occur in surgeries, meaning that any positive health impacts (as shown above) outweigh the negative. In addition, the safety of PRP is well established in the clinical trials discussed above, as is the safety of BMC. Specifically, Regenexx has published the world’s largest orthobiologics safety paper to date, and other authors have published similar data (57-59).
At Least Equivalent to the Established Treatments
Is IO at least the same in terms of outcomes as the current standard of care, which is orthopedic surgery? This question can be answered by examining how much evidence exists that orthopedic surgery is effective. Meaning is the bar for the existing technology set high or low? Regrettably, two different analyses have shown that almost all common orthopedic surgeries have very poor levels of supporting evidence (60, 61). For example, the chart below from the Blom paper referenced here is telling:
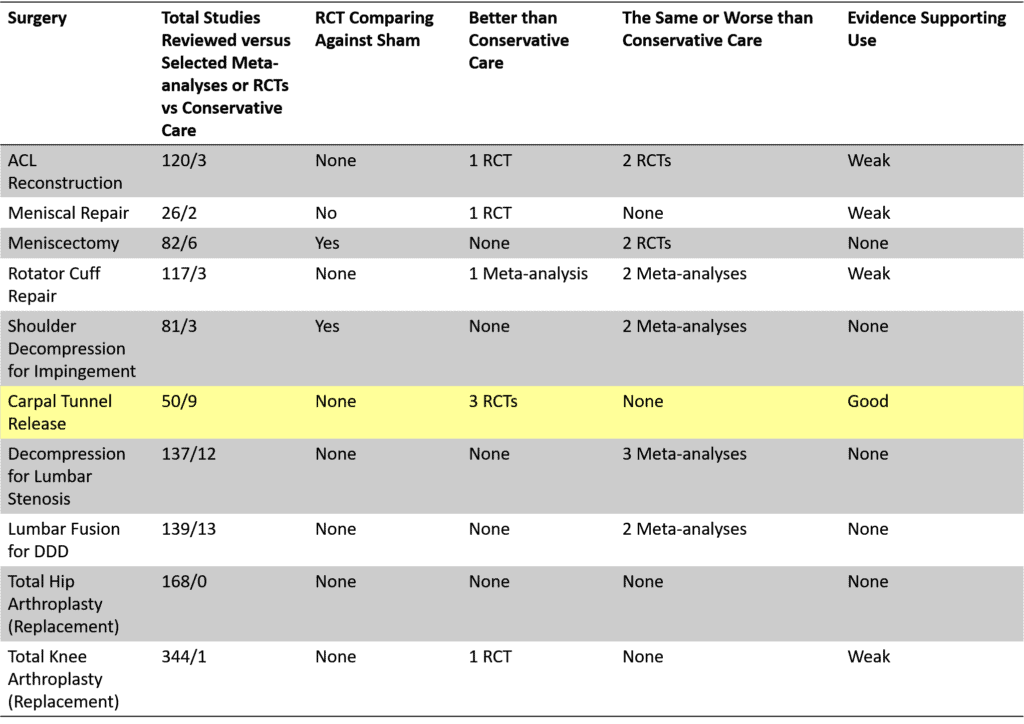
Of the ten most commonly performed procedures, only 1 (carpal tunnel surgery) can claim to have a reasonable level of RCT research showing it beats conservative care. Only two have ever been tested against a sham procedure, and both of those procedures (meniscectomy and shoulder decompression) were inferior.
If we go the other way and compare the outcomes of IO to surgery, data points are available. For example, Hernigou compared the use of BMC to treat knee osteoarthritis and found it to be superior to TKA at 15 years. (3) We compared our knee OA BMC procedure to TKA and found it to be as or more cost-effective using a QALY metric (62).
Finally, a new concept introduced with the advent of orthobiologics deserves discussion, which is “Surgical Avoidance”. This means that the purpose of many IO treatments is to allow the patient to avoid more invasive surgery or utilize it later in life. For example, the utility of a knee BMC injection is to avoid a TKA or allow the patient to avoid that procedure until an older age when the procedure is more likely to be a “one and done” rather than almost certain revision surgery. This is exactly what our knee BMC QALY study demonstrated (62).
Technology Available Outside of a Clinical Trial
Finally, the new investigational technology must be available outside of a clinical trial. Regenexx treatments have been used outside of a clinical trial since 2005.
In Summary
There are two distinct issues with adding an investigational technology to a health plan. The first is obvious. Are enough variables controlled to ensure that all sites are delivering the same technology in the same way and collecting outcomes? Second, can that technology clear the hurdles insurers use to ensure that it’s ready to be added to a health plan. Regenexx meets both criteria.
The upshot? It’s not hard to see why only Regenexx has been able to pull off being added to the health plans of more than 500 employers. One part of that equation is the type of standardization that is rarely found in the wild west of regenerative medicine. The second is the ability to clear the investigational standard used by insurers and TPAs.
__________________________________________________________________________
References:
(1) Centeno C, Sheinkop M, Dodson E, Stemper I, Williams C, Hyzy M, Ichim T, Freeman M. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med. 2018 Dec 13;16(1):355. doi: 10.1186/s12967-018-1736-8. PMID: 30545387; PMCID: PMC6293635.
(2) Hernigou P, Bouthors C, Bastard C, Flouzat Lachaniette CH, Rouard H, Dubory A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? A randomized study. Int Orthop. 2020 Jul 2. doi: 10.1007/s00264-020-04687-7. Epub ahead of print. PMID: 32617651.
(3) Hernigou P, Delambre J, Quiennec S, Poignard A. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: a prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int Orthop. 2020 Apr 23. doi: 10.1007/s00264-020-04571-4. Epub ahead of print. PMID: 32322943.
(4) Rosário DAV, Faleiro TB, Franco BAFM, Daltro GC, Marchetto R. COMPARISON BETWEEN CONCENTRATED BONE MARROW ASPIRATE AND CORTICOID IN GLUTEAL TENDINOPATHY. Acta Ortop Bras. 2021 Jan-Feb;29(1):26-29. doi: 10.1590/1413-785220212901236828. PMID: 33795965; PMCID: PMC7976861.
(5) Senna MK, Shaat RM, Ali AAA. Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin Rheumatol. 2019 Dec;38(12):3643-3654. doi: 10.1007/s10067-019-04719-7. Epub 2019 Aug 16. PMID: 31420812.
(6) Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013 Nov;41(11):2609-16. doi: 10.1177/0363546513496542. Epub 2013 Jul 26. PMID: 23893418.
(7) Malahias MA, Nikolaou VS, Johnson EO, Kaseta MK, Kazas ST, Babis GC. Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: A placebo-controlled clinical study. J Tissue Eng Regen Med. 2018 Mar;12(3):e1480-e1488. doi: 10.1002/term.2566. Epub 2017 Dec 17. PMID: 28873284.
(8) Malahias MA, Nikolaou VS, Johnson EO, Kaseta MK, Kazas ST, Babis GC. Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: A placebo-controlled clinical study. J Tissue Eng Regen Med. 2018 Mar;12(3):e1480-e1488. doi: 10.1002/term.2566. Epub 2017 Dec 17. PMID: 28873284.
(9) Uslu Güvendi E, Aşkin A, Güvendi G, Koçyiğit H. Comparison of Efficiency Between Corticosteroid and Platelet Rich Plasma Injection Therapies in Patients With Knee Osteoarthritis. Arch Rheumatol. 2017;33(3):273–281. Published 2017 Nov 2. doi: 10.5606/ArchRheumatol.2018.6608
(10) Tavassoli M, Janmohammadi N, Hosseini A, Khafri S, Esmaeilnejad-Ganji SM. Single- and double-dose of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: A randomized controlled trial. World J Orthop. 2019;10(9):310–326. Published 2019 Sep 18. doi: 10.5312/wjo.v10.i9.310
(11) Joshi Jubert N, Rodríguez L, Reverté-Vinaixa MM, Navarro A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop J Sports Med. 2017;5(2):2325967116689386. Published 2017 Feb 13. doi: 10.1177/2325967116689386
(12) Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) Versus Hyaluronic Acid (A one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. Published 2015 Jan 7. doi: 10.4137/CMAMD.S17894
(13) Montañez-Heredia E, Irízar S, Huertas PJ, et al. Intra-Articular Injections of Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System. Int J Mol Sci. 2016;17(7):1064. Published 2016 Jul 2. doi: 10.3390/ijms17071064
(14) Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017 Mar;25(3):958-965. doi: 10.1007/s00167-015-3705-6.
(15) Lana JF, Weglein A, Sampson SE, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69–78. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5227106/
(16) Tavassoli M, Janmohammadi N, Hosseini A, Khafri S, Esmaeilnejad-Ganji SM. Single- and double-dose of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: A randomized controlled trial. World J Orthop. 2019;10(9):310–326. Published 2019 Sep 18. doi: 10.5312/wjo.v10.i9.310
(17) Lin KY, Yang CC, Hsu CJ, Yeh ML, Renn JH. Intra-articular Injection of Platelet-Rich Plasma Is Superior to Hyaluronic Acid or Saline Solution in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized, Double-Blind, Triple-Parallel, Placebo-Controlled Clinical Trial. Arthroscopy. 2019 Jan;35(1):106-117. doi: 10.1016/j.arthro.2018.06.035.
(18) Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis : A prospective randomized controlled study. Orthopade. 2019 Mar;48(3):239-247. doi: 10.1007/s00132-018-03659-5.
(19) Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, Kon E, Filardo G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am J Sports Med. 2019 Feb;47(2):347-354. doi: 10.1177/0363546518814532.
(20) Yu W, Xu P, Huang G, Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med. 2018;16(3):2119–2125. doi: 10.3892/etm.2018.6412
(21) Buendía-López D, Medina-Quirós M, Fernández-Villacañas Marín MÁ. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol. 2018;19(1):3. Published 2018 Aug 20. doi: 10.1186/s10195-018-0501-3
(22) Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018 May;37(5):1341-1350. doi: 10.1007/s10067-018-3985-6.
(23) Louis ML, Magalon J, Jouve E, Bornet CE, Mattei JC, Chagnaud C, Rochwerger A, Veran J3, Sabatier F. Growth Factors Levels Determine Efficacy of Platelets Rich Plasma Injection in Knee Osteoarthritis: A Randomized Double Blind Noninferiority Trial Compared With Viscosupplementation. Arthroscopy. 2018 May;34(5):1530-1540.e2. doi: 10.1016/j.arthro.2017.11.035.
(24) Lisi C, Perotti C, Scudeller L, Sammarchi L, Dametti F, Musella V, Di Natali G. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil. 2018 Mar;32(3):330-339. doi: 10.1177/0269215517724193
(25) Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am J Sports Med. 2017 Feb;45(2):339-346. doi: 10.1177/0363546516665809.
(26) Kaminski R, Maksymowicz-Wleklik M, Kulinski K, Kozar-Kaminska K, Dabrowska-Thing A, Pomianowski S. Short-Term Outcomes of Percutaneous Trephination with a Platelet Rich Plasma Intrameniscal Injection for the Repair of Degenerative Meniscal Lesions. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. Int J Mol Sci. 2019 Feb 16;20(4):856. doi: 10.3390/ijms20040856. PMID: 30781461; PMCID: PMC6412887.
(27) Malahias MA, Roumeliotis L, Nikolaou VS, Chronopoulos E, Sourlas I, Babis GC. Platelet-Rich Plasma versus Corticosteroid Intra-Articular Injections for the Treatment of Trapeziometacarpal Arthritis: A Prospective Randomized Controlled Clinical Trial. Cartilage. 2021 Jan;12(1):51-61. doi: 10.1177/1947603518805230. Epub 2018 Oct 20. PMID: 30343590; PMCID: PMC7755966.
(28) Dallari D, Stagni C, Rani N, Sabbioni G, Pelotti P, Torricelli P, Tschon M, Giavaresi G. Ultrasound-Guided Injection of Platelet-Rich Plasma and Hyaluronic Acid, Separately and in Combination, for Hip Osteoarthritis: A Randomized Controlled Study. Am J Sports Med. 2016 Mar;44(3):664-71. doi: 10.1177/0363546515620383. Epub 2016 Jan 21. PMID: 26797697.
(29) Battaglia M, Guaraldi F, Vannini F, Rossi G, Timoncini A, Buda R, Giannini S. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013 Dec;36(12):e1501-8. doi: 10.3928/01477447-20131120-13. PMID: 24579221.
(30) Pasin T, Ataoğlu S, Pasin Ö, Ankarali H. Comparison of the Effectiveness of Platelet-Rich Plasma, Corticosteroid, and Physical Therapy in Subacromial Impingement Syndrome. Arch Rheumatol. 2019 Mar 28;34(3):308-316. doi: 10.5606/ArchRheumatol.2019.7225. PMID: 31598597; PMCID: PMC6768781.
(31) Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016 Dec;26(8):837-842. doi: 10.1007/s00590-016-1826-3. Epub 2016 Aug 20. PMID: 27544678.
(32) Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013 Nov;41(11):2609-16. doi: 10.1177/0363546513496542. Epub 2013 Jul 26. PMID: 23893418.
(33) Cai YU, Sun Z, Liao B, Song Z, Xiao T, Zhu P. Sodium Hyaluronate and Platelet-Rich Plasma for Partial-Thickness Rotator Cuff Tears. Med Sci Sports Exerc. 2019;51(2):227-233. doi:10.1249/MSS.0000000000001781
(34) Lin J. Platelet-rich plasma injection in the treatment of frozen shoulder: A randomized controlled trial with 6-month follow-up . Int J Clin Pharmacol Ther. 2018 Aug;56(8):366-371. doi: 10.5414/CP203262. PMID: 29932415.
(35) Nejati P, Ghahremaninia A, Naderi F, Gharibzadeh S, Mazaherinezhad A. Treatment of Subacromial Impingement Syndrome: Platelet-Rich Plasma or Exercise Therapy? A Randomized Controlled Trial. Orthop J Sports Med. 2017 May 19;5(5):2325967117702366. doi: 10.1177/2325967117702366. PMID: 28567426; PMCID: PMC5439655.
(36) Pasin T, Ataoğlu S, Pasin Ö, Ankarali H. Comparison of the Effectiveness of Platelet-Rich Plasma, Corticosteroid, and Physical Therapy in Subacromial Impingement Syndrome. Arch Rheumatol. 2019 Mar 28;34(3):308-316. doi: 10.5606/ArchRheumatol.2019.7225. PMID: 31598597; PMCID: PMC6768781.
(37) Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013 Feb;27(2):113-22. doi: 10.1177/0269215512448388. Epub 2012 Oct 3. PMID: 23035005.
(38) Senna MK, Shaat RM, Ali AAA. Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin Rheumatol. 2019 Dec;38(12):3643-3654. doi: 10.1007/s10067-019-04719-7. Epub 2019 Aug 16. PMID: 31420812.
(39) Pasin T, Ataoğlu S, Pasin Ö, Ankarali H. Comparison of the Effectiveness of Platelet-Rich Plasma, Corticosteroid, and Physical Therapy in Subacromial Impingement Syndrome. Arch Rheumatol. 2019 Mar 28;34(3):308-316. doi: 10.5606/ArchRheumatol.2019.7225. PMID: 31598597; PMCID: PMC6768781.
(40) Mishra AK, Skrepnik NV, Edwards SG, Jones GL, Sampson S, Vermillion DA, Ramsey ML, Karli DC, Rettig AC. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014 Feb;42(2):463-71. doi: 10.1177/0363546513494359. Epub 2013 Jul 3. PMID: 23825183.
(41) Pasin T, Ataoğlu S, Pasin Ö, Ankarali H. Comparison of the Effectiveness of Platelet-Rich Plasma, Corticosteroid, and Physical Therapy in Subacromial Impingement Syndrome. Arch Rheumatol. 2019 Mar 28;34(3):308-316. doi: 10.5606/ArchRheumatol.2019.7225. PMID: 31598597; PMCID: PMC6768781.
(42) Martínez-Montiel O, Valencia-Martinez G, Blanco-Bucio P, Villalobos-Campuzano C. Tratamiento de epicondilitis de codo con plasma rico en plaquetas versus corticosteroide local [Treatment of elbow epicondylitis with platelet rich plasma versus local corticosteroids]. Acta Ortop Mex. 2015 May-Jun;29(3):155-8. Spanish. PMID: 26999966.
(43) Palacio EP, Schiavetti RR, Kanematsu M, Ikeda TM, Mizobuchi RR, Galbiatti JA. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Rev Bras Ortop. 2016 Jan 13;51(1):90-5. doi: 10.1016/j.rboe.2015.03.014. PMID: 26962506; PMCID: PMC4767828.
(44) Pasin T, Ataoğlu S, Pasin Ö, Ankarali H. Comparison of the Effectiveness of Platelet-Rich Plasma, Corticosteroid, and Physical Therapy in Subacromial Impingement Syndrome. Arch Rheumatol. 2019 Mar 28;34(3):308-316. doi: 10.5606/ArchRheumatol.2019.7225. PMID: 31598597; PMCID: PMC6768781.
(45) Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015 Apr;23(1):1-5. doi: 10.1177/230949901502300101. PMID: 25920633.
(46) Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011 Jun;39(6):1200-8. doi: 10.1177/0363546510397173. Epub 2011 Mar 21. PMID: 21422467.
(47) Merolla G, Dellabiancia F, Ricci A, Mussoni MP, Nucci S, Zanoli G, Paladini P, Porcellini G. Arthroscopic Debridement Versus Platelet-Rich Plasma Injection: A Prospective, Randomized, Comparative Study of Chronic Lateral Epicondylitis With a Nearly 2-Year Follow-Up. Arthroscopy. 2017 Jul;33(7):1320-1329. doi: 10.1016/j.arthro.2017.02.009. Epub 2017 Apr 19. PMID: 28433443.
(48) Raeissadat SA, Rayegani SM, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is Platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014 Mar 18;6:12. doi: 10.1186/2052-1847-6-12. PMID: 24635909; PMCID: PMC4006635.
(49) Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011 Oct;39(10):2130-4. doi: 10.1177/0363546511417113. Epub 2011 Aug 2. PMID: 21813443.
(50) Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013 Nov;41(11):2609-16. doi: 10.1177/0363546513496542. Epub 2013 Jul 26. PMID: 23893418.
(51) Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013 Nov;41(11):2609-16. doi: 10.1177/0363546513496542. Epub 2013 Jul 26. PMID: 23893418.
(52) Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013 Nov;41(11):2609-16. doi: 10.1177/0363546513496542. Epub 2013 Jul 26. PMID: 23893418.
(53) Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H. Effect of High-Volume Injection, Platelet-Rich Plasma, and Sham Treatment in Chronic Midportion Achilles Tendinopathy: A Randomized Double-Blinded Prospective Study. Am J Sports Med. 2017 Jul;45(9):2034-2043. doi: 10.1177/0363546517702862. Epub 2017 May 22. PMID: 28530451.
(54) Alsousou J, Thompson M, Harrison P, Willett K, Franklin S. Effect of platelet-rich plasma on healing tissues in acute ruptured Achilles tendon: a human immunohistochemistry study. Lancet. 2015 Feb 26;385 Suppl 1:S19. doi: 10.1016/S0140-6736(15)60334-8. PMID: 26312841.
(55) Berger DR, Centeno CJ, Steinmetz NJ. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Joint Res. 2019 Feb 2;8(1):32-40. doi: 10.1302/2046-3758.81.BJR-2018-0164.R1. PMID: 30800297; PMCID: PMC6359887.
(56) Maruenda JI, Barrios C, Garibo F, Maruenda B. Adjacent segment degeneration and revision surgery after circumferential lumbar fusion: outcomes throughout 15 years of follow-up. Eur Spine J. 2016 May;25(5):1550-1557. doi: 10.1007/s00586-016-4469-5. Epub 2016 Mar 8. PMID: 26957098.
(57) Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Aug;40(8):1755-1765. doi: 10.1007/s00264-016-3162-y. Epub 2016 Mar 30. Erratum in: Int Orthop. 2018 Jan;42(1):223. PMID: 27026621.
(58) Hernigou P, Homma Y, Flouzat-Lachaniette CH, Poignard A, Chevallier N, Rouard H. Cancer risk is not increased in patients treated for orthopaedic diseases with autologous bone marrow cell concentrate. J Bone Joint Surg Am. 2013 Dec 18;95(24):2215-21. doi: 10.2106/JBJS.M.00261. PMID: 24352775.
(59) Hernigou P, Flouzat Lachaniette CH, Delambre J, Chevallier N, Rouard H. Regenerative therapy with mesenchymal stem cells at the site of malignant primary bone tumour resection: what are the risks of early or late local recurrence? Int Orthop. 2014 Sep;38(9):1825-35. doi: 10.1007/s00264-014-2384-0. Epub 2014 Jun 7. PMID: 24906983.
(60) Lohmander LS, Roos EM. The evidence base for orthopaedics and sports medicine. BMJ. 2015 Jan 2;350:g7835. doi: 10.1136/bmj.g7835. PMID: 25555826.
(61) Blom AW, Donovan RL, Beswick AD, Whitehouse MR, Kunutsor SK. Common elective orthopaedic procedures and their clinical effectiveness: umbrella review of level 1 evidence. BMJ. 2021 Jul 7;374:n1511. doi: 10.1136/bmj.n1511. PMID: 34233885.
(62) Centeno C. QALY Analysis of BMC Treatment for Knee OA vs TKR. The Interventional Orthobiologics Foundation 2022 Annual Conference. Denver, Colorado. See https://app.box.com/s/jt8376x35n7jxvy63w5kyzq3wd2tebki
(63) Manchikanti L, Centeno CJ, Atluri S, Albers SL, Shapiro S, Malanga GA, Abd-Elsayed A, Jerome M, Hirsch JA, Kaye AD, Aydin SM, Beall D, Buford D, Borg-Stein J, Buenaventura RM, Cabaret JA, Calodney AK, Candido KD, Cartier C, Latchaw R, Diwan S, Dodson E, Fausel Z, Fredericson M, Gharibo CG, Gupta M, Kaye AM, Knezevic NN, Kosanovic R, Lucas M, Manchikanti MV, Mason RA, Mautner K, Murala S, Navani A, Pampati V, Pastoriza S, Pasupuleti R, Philip C, Sanapati MR, Sand T, Shah RV, Soin A, Stemper I, Wargo BW, Hernigou P. Bone Marrow Concentrate (BMC) Therapy in Musculoskeletal Disorders: Evidence-Based Policy Position Statement of American Society of Interventional Pain Physicians (ASIPP). Pain Physician. 2020 Mar;23(2):E85-E131. PMID: 32214287.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
