How Not Using a Treatment Registry May Blow Up Your Practice
Credit: Shutterstock
This week I got a call from a medical provider who is being investigated by his state attorney general over a dissatisfied patient. IMHO that provider is at higher risk because he didn’t use a treatment registry. So let’s dig into why not tracking your outcomes when using interventional orthobiologics may risk your entire practice.
The Seatbelt Analogy
Do you wear your seatbelt? After all, if you never wear it in any given year, your chance of serious injury in a car crash is reasonably low. However, it’s that one time you need it that you realize that not developing that habit was a bad idea. That is if you live to tell the tale.
If you’re practicing interventional orthobiologics, a patient registry is your seatbelt. In addition, it’s a major way to be transparent with your patients. Finally, it’s the only way to see who is a good or bad candidate for what you do so that you can be honest and upfront with patients.
What is a Patient Treatment Registry?
A patient treatment registry is a system that tracks patient-reported outcomes and complications. This process is commonly used to track knee replacement patients. For interventional orthobiologics, a registry allows a practice to track how patients are doing and whether a particular procedure in a specific patient type, severity, or disease is worth performing or avoiding.
How does a registry change your practice? Let’s look at an example. We have long noted in our registry data that bone marrow concentrate (BMC) used to treat more severe hip arthritis is ineffective. We know this because Regenexx runs the world’s largest and oldest orthobiologics registry, and we analyzed and published this observation long ago (5). Hence, when we see a patient with severe hip arthritis, we tell those patients that they are poor candidates. This helps the patient understand that if they choose to get a BMC procedure, their likelihood of avoiding a hip replacement is low. Finally, in my clinical experience, most patients confronted with that data chose to get a hip replacement and skip the BMC injection.
Why is a Registry Critical in Interventional Orthobiologics?
While more and more of interventional orthobiologics (IO) (like PRP use in knee osteoarthritis) is being accepted by mainstream orthopedics, most of what the average IO provider does on a day-to-day basis is still investigational. Hence, that means tracking outcomes and complications is critical. Why? Because the basic questions of efficacy and sometimes even long-term safety have yet to be answered.
Let’s give a complications example where we recently used registry data to answer a very important question. We know that blood clots like DVT and PE are a real risk in knee replacement surgery and that mitigating this risk requires anticoagulant drugs. However, what is the risk of blood clots when a knee bone marrow concentrate procedure is used? Is that risk more or less than the risk of excessive bleeding when taking these drugs? Our analysis of more than 12,000 procedures showed there was no need to routinely anticoagulate these patients (1).
What Do the Academics and Other IO Experts Say?
A few years back, I convened a Delphi panel of academic and other experts in the field. The goal was to use the Delphi process to reach a consensus about the standard of care for BMC procedures. Those results were published, and the experts agreed upon the following (6):
“Areas of agreement included importance of a treatment registry, candidacy grading…”.
Why Providers Who Don’t Track Outcomes are an Accident Waiting to Happen
I got a call from a medical provider being investigated by the state attorney general’s office. This is based on a patient who didn’t get much relief from her shoulder arthritis symptoms despite a BMC/MFat/PRP injection procedure. Several years ago, the patient took the provider to small claims court, and the judge threw the case out. A medical board complaint was also dismissed. However, 1-2 years later, a second medical board complaint wasn’t dismissed and was referred to the state attorney general’s office. My first question for the provider was, do you have outcome data from the registry you have been using? The answer was that he never collected any data.
How would his defense be VERY different if this provider had collected outcome data on this specific procedure and clinical indication via a registry? First, he could point to many procedures showing that a reasonably high percentage of patients improved and that this patient was simply in the group that failed to respond. The procedure was justified because there was a reasonable expectation of success, and the odds of treatment failure were disclosed to the patient.
How would this have been different if this happened to a Regenexx provider? First, our shoulder outcomes data is published online here, so this patient would have had easy access to this information. Hence, there is complete transparency from the beginning, with the patient understanding that our procedure is NOT magic. This is from that page today, which shows the results of 2,636 BMC-treated shoulder patients:
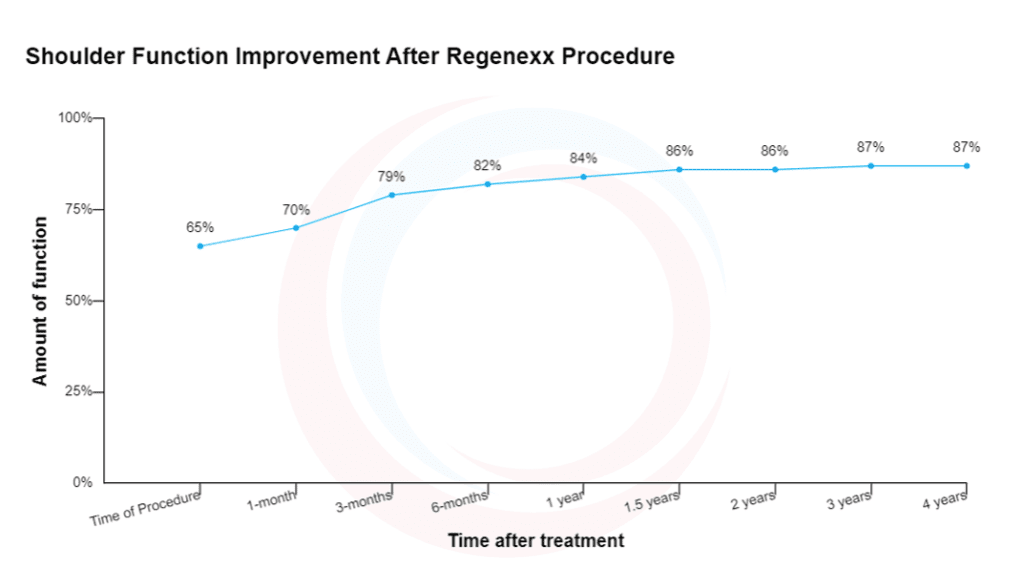
This graph shows a nice improvement in shoulder function for these patients. However, the average doesn’t get to 100% function. This means that there are patients in this dataset who underperform and outperform the average. Next, we have also published this specific shoulder data (2). Our other published studies have highlighted these shoulder procedure results differently (3,4). Finally, our research team’s separate analysis of this shoulder registry data and MRIs in osteoarthritis patients shows that patients with certain MRI findings have a poor response to BMC procedures. Hence, we can inform those patients that they are less likely to respond and then let them decide on whether to proceed.
In summary, defending a doctor’s specific IO procedure as reasonable, given the lack of medical evidence, is very tough to do without this type of registry data. That puts medical providers at risk for things like a medical board disciplinary action or even a successful medical malpractice claim.
Using a Registry
If you’re a provider practicing interventional orthobiologics and not using a registry to both protect yourself and provide transparency to your patients about what works and doesn’t, in my opinion, you’re practicing BELOW the standard of care. What if you have no plan to join the Regenexx network? You can use open registries, like the one founded by the late great Gerry Malanga (DataBiologics). However, the worst choice is to forego collecting data because it’s too big a pain.
The upshot? A patient registry is like wearing your seatbelt. You never know when it’s going to come in handy. Driving without your seatbelt is not smart. Practicing interventional orthobiologics without using a patient registry is just as risky.
________________________________________________
References:
(1) Centeno, C.J., Money, B.T., Dodson, E. et al. The rate of venous thromboembolism after knee bone marrow concentrate procedures: should we anticoagulate?. International Orthopaedics (SICOT) 46, 2213–2218 (2022). https://doi.org/10.1007/s00264-022-05500-3
(2) Centeno et al. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. Journal of Pain Research 2015:8 269–276.
(3) Christopher Centeno, Zachary Fausel, Ian Stemper, Ugochi Azuike, Ehren Dodson, “A Randomized Controlled Trial of the Treatment of Rotator Cuff Tears with Bone Marrow Concentrate and Platelet Products Compared to Exercise Therapy: A Midterm Analysis”, Stem Cells International, vol. 2020, Article ID 5962354, 10 pages, 2020. https://doi.org/10.1155/2020/5962354
(4) Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Aug;40(8):1755-1765. doi: 10.1007/s00264-016-3162-y. Epub 2016 Mar 30. Erratum in: Int Orthop. 2018 Jan;42(1):223. PMID: 27026621.
(5) Centeno et al. Efficacy and Safety of Bone Marrow Concentrate for Osteoarthritis of the Hip; Treatment Registry Results for 196 Patients. J Stem Cell Res Ther 2014, 4:10
(6) Centeno CJ, Jerome MA, Pastoriza SM, Shapiro S, Mautner K, Malanga GA, DePalma MJ, Mason RA, Stemper I, Dodson E. Use of Bone Marrow Concentrate to Treat Pain and Musculoskeletal Disorders: An Academic Delphi Investigation. Pain Physician. 2021 May;24(3):263-273. PMID: 33988946.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
