Is PRP a DMOAD? The Influence of Platelet Concentration on Study Failure
The holy grail of osteoarthritis research has long been a Disease Modifying OsteoArthritis Drug or DMOAD. This is a medication you can take or inject that would slow or prevent the onset of wear and tear arthritis. A big question for doctors who have observed the power of orthobiologics is whether PRP is a DMOAD. The answer is likely based on whether the study used real or fake PRP. Let’s dig in.
What Is a DMOAD?
This is the Wikipedia definition (1):
“A disease-modifying osteoarthritis drug (DMOAD) is a disease-modifying drug that would inhibit or even reverse the progression of osteoarthritis. Since the main hallmark of osteoarthritis is cartilage loss, a typical DMOAD would prevent the loss of cartilage and potentially regenerate it. Other DMOADs may attempt to help repair adjacent tissues by reducing inflammation. A successful DMOAD would be expected to show an improvement in patient pain and function with an improvement of the health of the joint tissues.”
Older PRP DMOAD Research?
So, what’s been published on PRP as a DMOAD? In animal studies, PRP reduces the loss of cartilage (4). However, does that translate to the clinic?
A study that used PRP did demonstrate that it could reduce cartilage loss, but the authors reported that their data showed that the effects of this treatment were likely dependent on making sure platelet concentrations were high (3). Two other clinical research studies have also demonstrated that PRP improved or helped cartilage repair processes (2,6).
However, some studies have shown that while PRP improved pain and function, there was no change in cartilage thickness or loss over time (5,7). However, could these investigations have suffered from a problem I have previously pointed out in other PRP research?
Fake PRP Studies
Some studies that claim to have used PRP didn’t actually meet the 2X definition of PRP as defined by Marx (8). This original definition of Platelet-rich Plasma was that the platelets in the mix were concentrated at least two times over their baseline concentration in whole blood. Despite this clear delineation, we’ve seen several major studies published in big journals test plasma that was too poor in platelets to surpass this very low bar.
Analyzing All PRP DMOAD Studies
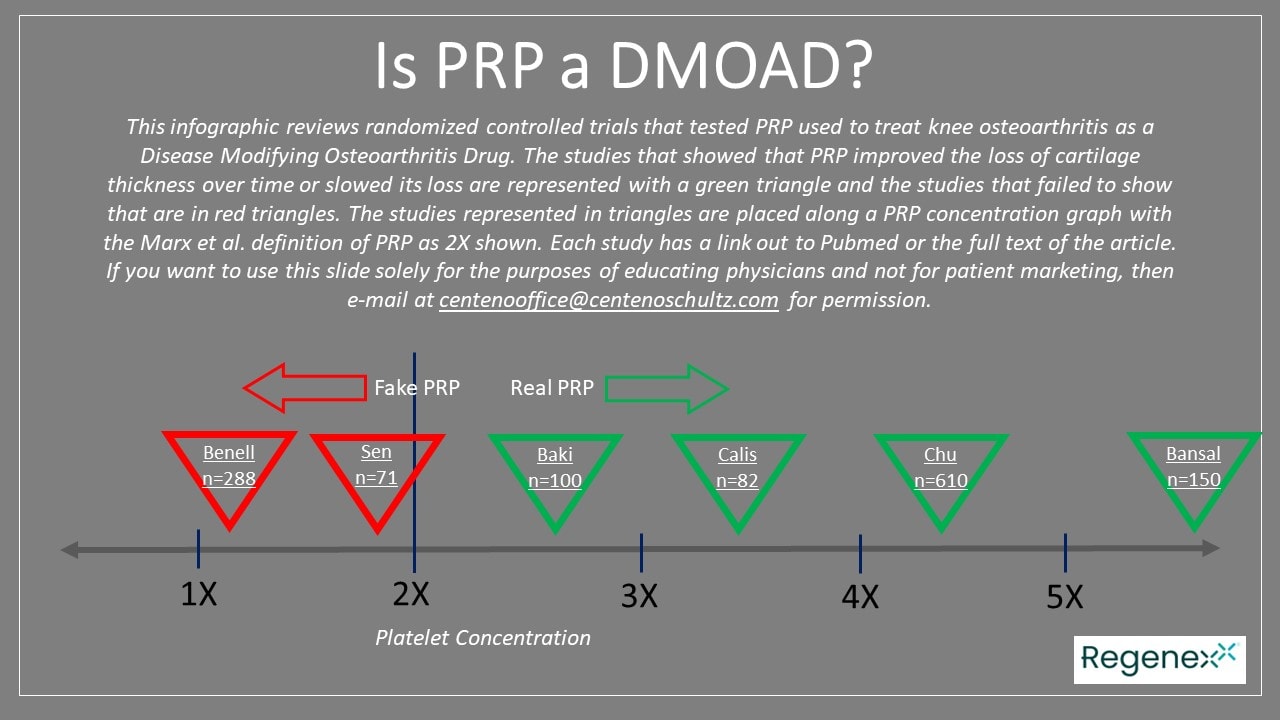
At the top of the page, you will see an infographic I created with the available PRP knee arthritis studies that I could find on Pubmed. These tested this injection versus a placebo and then looked at cartilage thickness over time. Each triangle represents a study and has a link to that research. The red triangles represent that the authors found no DMOAD effect, and the green triangles signify that the authors did find a DMOAD effect. Each triangle is then placed along a PRP concentration graph showing whether it used less than or more than the minimum definition of PRP.
An Analysis of the Concentration of the Two Failed PRP DMOAD Studies
Two of the 6 PRP DMOAD studies I found didn’t show a DMOAD effect. Let’s deconstruct these two research studies regarding the concentration of PRP used by the authors. After all, it’s reasonable to ask whether these study failures could have been because the plasma used was too poor in platelets to meet the minimum PRP definition.
First is Şen Eİ et al., which has some truly funny PRP math. The authors used ultrasound imaging of the medial femoral condyle. They report that they drew 18 ml of whole blood and ended up with PRP with an 8-10X concentration. However, that’s impossible, given that they claim to have injected 4 ml. How concentrated could this have been? With 100% platelet capture efficiency, which is impossible, at most, it could have been 18/4, which is 4.5X. Given that most machines and systems have about a 29-55% efficiency, if we take the middle of those two numbers and apply a 42% efficiency, we get 4.5 x 0.42 or 1.89X (9). Hence, this study is unlikely to have met the minimum definition of PRP. How much blood would they have had to draw to get to a 10X PRP? A whopping 142 ml of blood and not the tiny 18 ml drawn.
How about the second study? This is the infamous Benell et al. study I have covered before. They showed no cartilage thickness change on MRI, but these authors also never used actual PRP. The kit they used was capable of achieving only 1.6X, and the PRP characterization data reported showed even less concentration.
An Analysis of the Several Positive Prior PRP DMOAD Studies
The goal of this analysis is to show that based on blood volumes drawn and injected and realistic platelet recovery numbers, the PRP used was at least 2X or greater. Four studies did show a DMOAD effect, with three of these being older and one from last year, which is discussed separately below.
For example, the 2015 Calis et al. study took 24 ml of blood, which produced 3 ml of PRP. At our average 42% platelet capture efficiency, that would produce a real PRP at 3.4X. Even if we use the lowest PRP isolation efficiency published by Magalon et al. of 29%, the concentration is still at 2.3X.
The 2021 Baki study used a 30 ml blood draw and ended up with 2-4 ml of PRP and characterized their sample. The author’s analysis showed a mean platelet concentration of 2.6, meeting the Marx definition for PRP. That is certainly plausible given the blood volumes reported and a likely low platelet isolation efficiency of 26%.
The 2021 Bansal study used a much higher volume of a 60 ml blood draw, and they also characterized the sample. The final injectate was diluted so that each injection had approximately 10 billion platelets with an average concentration of 5.6X.
A Newer PRP DMOAD Study
The newer 2022 study that I’ll review in more detail looked at 610 patients at nine sites randomized to PRP or saline. PRP was injected three times (10). The PRP group separated well from the saline group for pain and function, with better cytokine profiles in the synovial fluid. More importantly, over five years, the PRP group had much lower cartilage loss (by less than half) when compared to saline.
How concentrated was this PRP? The authors claimed a 4.3X. Is that plausible? They took 50 ml of blood, which produced 5 ml of injectable PRP. A 4.3X would mean a 43% platelet collection efficiency, which is reasonable.
Conclusions?
If we use the longstanding Marx definition of PRP as the cutoff point, it’s clear that we have mounting evidence that PRP is a DMOAD. The two negative studies are tied to the same effect I reported earlier. In that more extensive analysis of almost 100 RCTs published on PRP, if the concentration was below 2X (meaning the authors used fake PRP), there was a 12 times greater risk of study failure.
More PRP Concentration?
All of these PRP concentrations are low compared to what most PRP experts use today. For example, at Regenexx, based on our published research and that from other authors, we routinely use 7-20X concentrations adjusted for age (older patients getting higher concentrations). Other colleagues routinely use 7-10X. Based on the available data, this likely reduces the individual failure rate of any given patient (11,12).
The upshot? If we filter out the few below-threshold PRP studies, we have mounting evidence that PRP is a DMOAD. That’s pretty exciting news, as this is the first treatment worldwide to show that effect based on high-level RCTs. Hence, I can give a firm recommendation now to my patients that a PRP knee injection will more likely than not help to protect the cartilage they have.
_________________________________________________
References:
(1) Wikipedia. Disease-modifying osteoarthritis drug. https://en.wikipedia.org/wiki/Disease-modifying_osteoarthritis_drug Accessed 8/27/23.
(2) Çalış, Havva Talay et al. “Efficacy of Intra-Articular Autologous Platelet Rich Plasma Application in Knee Osteoarthritis.” Archives of Rheumatology 30 (2015): 198-205.
(3) Bansal, H., Leon, J., Pont, J.L. et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci Rep 11, 3971 (2021). https://doi.org/10.1038/s41598-021-83025-2
(4) Asjid R, Faisal T, Qamar K, Khan SA, Khalil A, Zia MS. Platelet-rich Plasma-induced Inhibition of Chondrocyte Apoptosis Directly Affects Cartilage Thickness in Osteoarthritis. Cureus. 2019;11(11):e6050. Published 2019 Nov 1. doi:10.7759/cureus.6050
(5) Şen Eİ, Yıldırım MA, Yeşilyurt T, Kesiktaş FN, Dıraçoğlu D. Effects of platelet-rich plasma on the clinical outcomes and cartilage thickness in patients with knee osteoarthritis. J Back Musculoskelet Rehabil. 2020;33(4):597-605. doi: 10.3233/BMR-181209. PMID: 31594201.
(6) Baki M. Abdel Noha ,Nawito O. Zeinab ,Abdelsalam M. S. Nehal ,Sabry Dina , Elashmawy Hossam,Seleem A. Nagy ,Taha Ali Abdel-azeem Azza , El Ghobashy Mohamed, “Does Intra-Articular Injection of Platelet-Rich Plasma Have an Effect on Cartilage Thickness in Patients with Primary Knee Osteoarthritis?”, Current Rheumatology Reviews 2021; 17(3) . https://doi.org/10.2174/1573397117666210114151701
(7) Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A, Harris A, Yu SP, Connell D, Linklater J, Wang BH, Oo WM, Hunter DJ. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA. 2021 Nov 23;326(20):2021-2030. doi: 10.1001/jama.2021.19415. PMID: 34812863; PMCID: PMC8611484.
(8) Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004 Apr;62(4):489-96. doi: 10.1016/j.joms.2003.12.003. PMID: 15085519.
(9) Magalon J, Bausset O, Serratrice N, Giraudo L, Aboudou H, Veran J, Magalon G, Dignat-Georges F, Sabatier F. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014 May;30(5):629-38. doi: 10.1016/j.arthro.2014.02.020. PMID: 24725317.
(10) Chu J, Duan W, Yu Z, Tao T, Xu J, Ma Q, Zhao L, Guo JJ. Intra-articular injections of platelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2022 Dec;30(12):4063-4071. doi: 10.1007/s00167-022-06887-7. Epub 2022 Feb 6. PMID: 35124707.
(11) Berger DR, Centeno CJ, Steinmetz NJ. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Joint Res. 2019 Feb 2;8(1):32-40. doi: 10.1302/2046-3758.81.BJR-2018-0164.R1. PMID: 30800297; PMCID: PMC6359887.
(12) Everts PA, Lana JF, Onishi K, Buford D, Peng J, Mahmood A, Fonseca LF, van Zundert A, Podesta L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines. 2023; 11(7):1922. https://doi.org/10.3390/biomedicines11071922

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.