Liveyon: It’s Hard to Keep Up with the Spin…
You may have noticed that I recently shared lab test results that demonstrated that 5 common umbilical cord products whose manufacturer’s tout loads of stem cells, had no stem cells. One of those companies called Liveyon is now trying to spin that result, hence this blog. Let’s dig in.
Past Research on the MSC Content of Umbilical Cord Products
Right now, we have three independent labs that all tried to find mesenchymal stem cells in amniotic and umbilical cord products (1-3). All three labs including Cornell, UC Davis, and ours failed to find any MSCs, despite the claims of manufacturers and clinicians that they are stem cell rich products. Hence, as a fourth lab, the CSU Translational Medicine Institute was asked to test 5 commonly used umbilical cord products using one of the criteria for the ISCT guidelines to identify MSCs (4). That is that the cells must be plastic adherent and expand in culture to form colonies. That test is called a CFU-f test (Colony Forming Unit-fibroblast).
The Comment on My Blog
I recently saw a comment on my Umbilical Cord Stem Cell Therapy for Knees blog that piqued my interest:
“I spoke to the founder of LivYon. He said this test is invalid. He said the 6-well culture plates on the five common umbilical cord products did not have the proper medium for the culture to grow in. He said the problem was that they used a medium used only for bone marrow MSC to grow and that is why there are no living stem cells in any of the 5 umbilical cord products tested. “LivYon’s lab tests show an abundance of growth”. He also suggested that there are many studies show “significant” efficacy using cord blood. Please reply.”
First, this comment was directed toward the CSU testing described above. Those test results are below and as you see, they were disappointing at best:
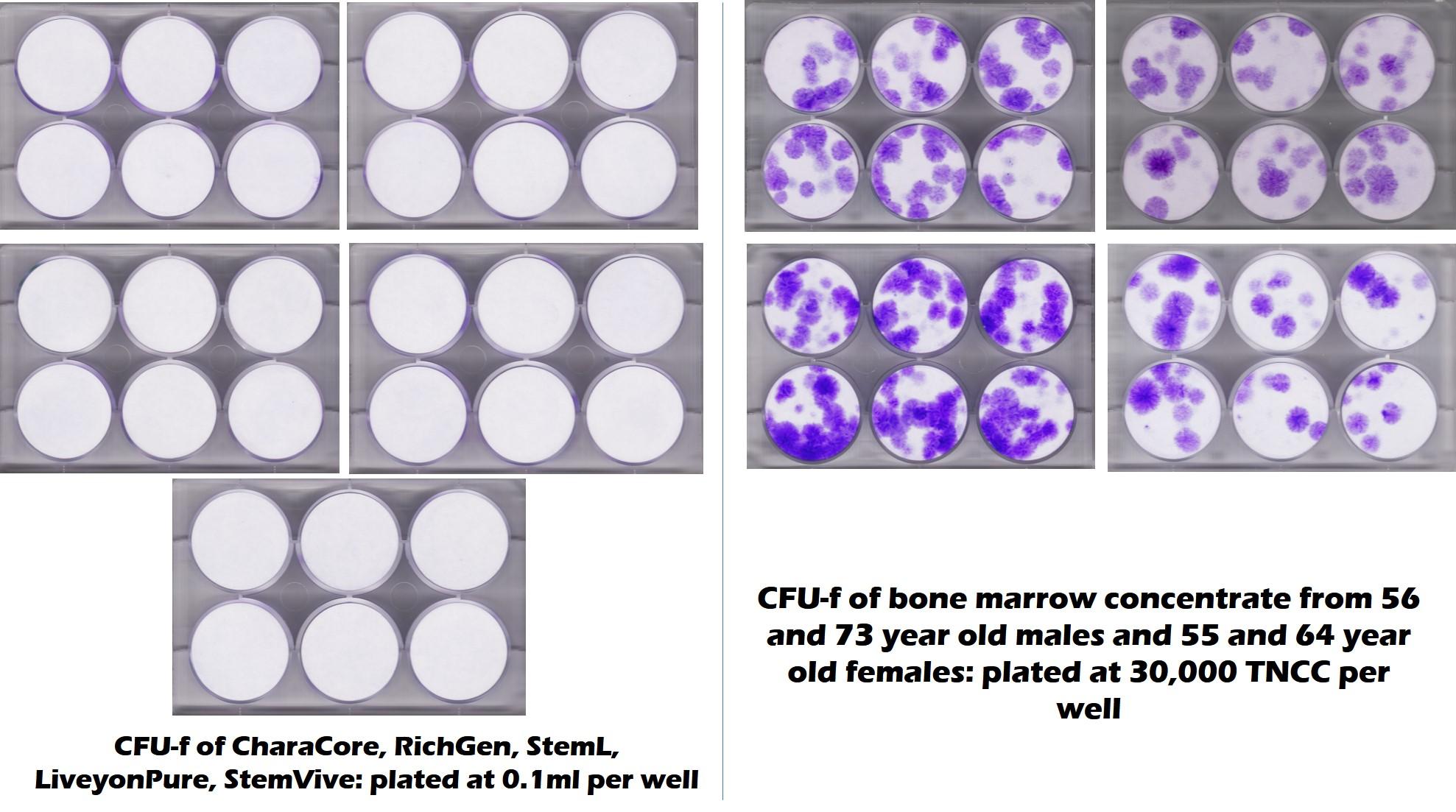
The purple dots on the CFU-f plates in the picture on the right are MSC colonies derived from middle-aged and elderly bone marrow. The plates on the left that have no purple dots are the 5 commonly used umbilical cord products that show no MSC colonies. The conclusion? These umbilical cord products have no MSCs.
Getting back to the comment left on my blog, there are two points here being made:
- CSU didn’t see any MSC colonies in the Liveyon umbilical cord blood product because they used the wrong culture media
- When the right culture media is used their product has a multitude of MSCs.
What is the Standard Culture Media to Grow MSCs from Umbilical Cord Blood?
If you search the word “Liveyon” on the search feature for this blog you will see many articles where the company has thrown out marketing collateral that looked scientific, but that was easily debunked by someone who knows stem cell biology. So let’s check this most recent claim that the wrong culture media was used. What even is culture media?
Culture media is the stuff that scientists use to grow cells (5). MSCs are grown in monolayer culture, which means they are placed into a flat culture flask or 6-well plate (like the ones shown above). They adhere to the bottom of the plate or flask and the culture media floats above. This is their “food”. The claim here is that CSU gave the MSCs in LiveyonPURE the wrong food, so they couldn’t grow, hence this is a false negative result.
What did CSU use? They used the gold standard for growing mesenchymal stem cells which is Fetal Bovine Serum or FBS (6).
However, what does the literature say is the correct media? Here’s what I found in one recent publication about growing umbilical cord MSCs:
“Almost all protocols use fetal bovine serum (FBS) as a supplement in culture media as a source of hormones, attachment factors and other molecules required for isolation and expansion of MSCs in both research and clinical fields.” (8)
If we look at several studies, here’s what we find is being used to grow MSCs from umbilical cords:
- Fetal Bovine Serum (7, 8, 9, 10, 11)
- Pooled platelet lysate (9, 11)
- Umbilical cord serum (8, 9, 10)
In summary, the idea that you can’t grow MSCs from umbilical cords in FBS is total fiction.
It Takes a Second to Spin a Falsehood and Hours to Debunk It
As we have seen many times from Liveyon and many other birth tissue vendors who claim to sell medical providers live and functional stem cells, they often make pseudoscientific claims in seconds that take a few hours to debunk, which is the big problem here. The owners of these companies know that the physicians, chiropractors, naturopaths, acupuncturists, and others buying this stuff have no idea which end is up when it comes to stem cells. Hence, they can simply spin whatever they want and as long as it sounds like science, these providers will buy it.
The upshot? The Liveyon claim that the reason CSU didn’t find MSCs in LiveyonPURE was because the lab used the wrong culture media is fiction. FBS has been a standard culture media used to grow MSCs from umbilical cords for decades. Hence CSU didn’t find any stem cells in their CFU-f tests becuase there were no viable and functional MSCs in the LiveyonPURE product samples tested.
_________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. https://www.ncbi.nlm.nih.gov/pubmed/16923606
(5) Bieback K, Kinzebach S, Karagianni M. Translating research into clinical scale manufacturing of mesenchymal stromal cells. Stem Cells Int. 2011;2010:193519. Published 2011 Jan 20. doi:10.4061/2010/193519
(6) Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014 Feb;16(2):170-80. doi: 10.1016/j.jcyt.2013.11.004.
(7) Qiang A, Juan X, Yanqiu Y, Gengsheng M, Qingyan Z, Wenyong G, Hongyun H. Standards for the culture and quality control of umbilical cord mesenchymal stromal cells for neurorestorative clinical application (2017). Journal of Neurorestoratology . 2018, (1): 11 -15 . DOI: 10.2147/JN.S148686
(8) Hassan G, Kasem I, Soukkarieh C, Aljamali M. A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum. Int J Stem Cells. 2017;10(2):184–192. doi: 10.15283/ijsc17028
(9) Hassan G, Kasem I, Antaki R, Bahjat Mohammad M, AlKadry R,
Aljamali M. Isolation of umbilical cord mesenchymal stem cells using human blood derivatives accompanied with explant method. Stem Cell Investig 2019;6:28. http://dx.doi.org/10.21037/sci.2019.08.06
(10) Shetty P, Bharucha K, Tanavde V. Human umbilical cord blood serum can replace fetal bovine serum in the culture of mesenchymal stem cells. Cell Biol Int. 2007 Mar;31(3):293-8. Epub 2006 Nov 19. https://www.ncbi.nlm.nih.gov/pubmed/17208468
(11) Kandoi S, L PK, Patra B, Vidyasekar P, Sivanesan D, Verma RS. Evaluation of platelet lysate as a substitute for FBS in explant and enzymatic isolation methods of human umbilical cord MSCs. Sci Rep. 2018 Aug 20;8(1):12439. doi: 10.1038/s41598-018-30772-4.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
