mVASC is Ground Up Cadaver SubQ Tissue? Huh?
Sometimes, you really can’t make stuff up in this field. This weekend, a colleague emailed me asking if I knew anything about mVasc, as one of his patients had been told it could effectively treat knee and ankle arthritis. I had never heard of this tissue, so I looked it up. It turns out it’s ground-up cadaver subQ tissue. After an analysis of this stuff, I’m still scratching my head. Let’s dive in.
What the Heck is mVASC?
mVASC is an allogeneic tissue product produced by a San Diego company called MicroVascular Tissues. This is from the website:
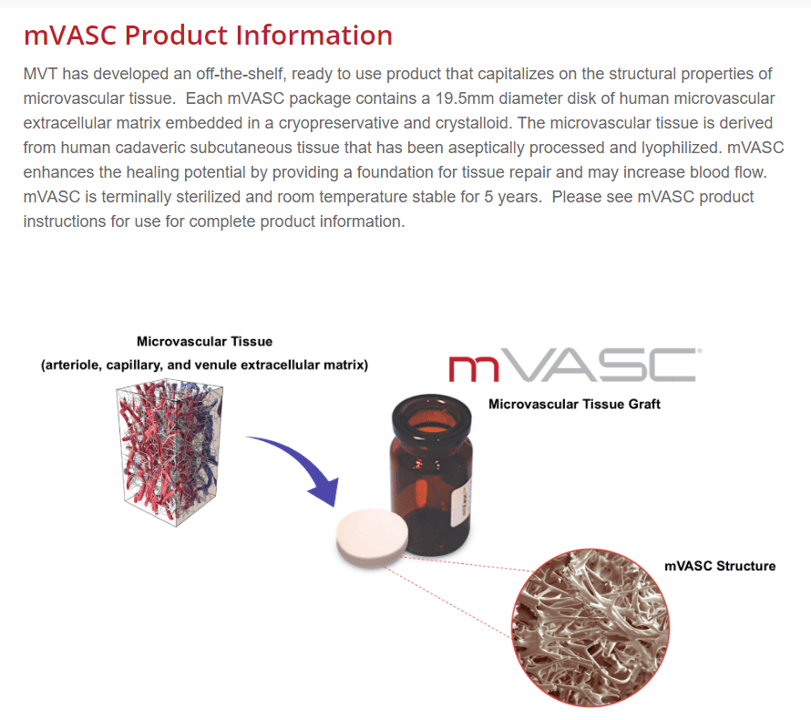
So basically, the company takes subcutaneous tissue from cadavers and lyophilizes it. Given that the patient wanted this injected into his joints and that the company has a white paper case study on a knee arthritis patient, I will have to assume that once you reconstitute it, it’s an injectable mush.
Using mVASC for Knee OA?
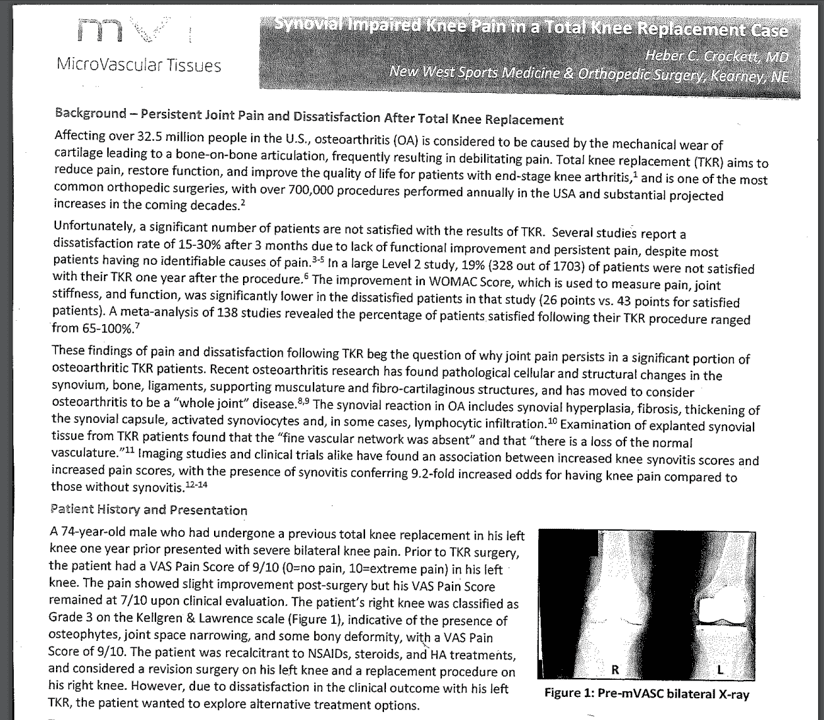
My colleague sent me the above flyer that was given to his patient. It’s a case study of a single patient with KL 3 knee OA injected with mVASC. The substance was reconstituted with three cc 0.25% Marcaine, and the mVASC solution was injected into each knee. These were the reported results:
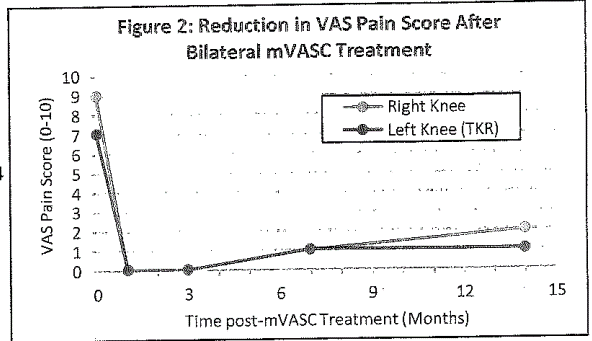
The graph above shows reported pain relief.
Other mVASC Research
I searched the US National Library of Medicine (Pubmed) for peer-reviewed and published mVASC articles. I found a single paper published in 2020 on wound healing (1). The in-vitro data and animal model focused on the angiogenic properties of mVASC. The clinical study was a small case series of three non-healing wounds treated with mVASC.
PRP Much?
When my colleague told me the mVASC website had convinced a patient of his to get his knee and ankle arthritis treated with this stuff, I have to admit that, knowing what I know about Regenerative Medicine, I laughed out loud. Cadaver subQ tissue? Really? After reviewing the information, I’m still scratching my head. However, all of this is a great example of how low information patients can get swayed by a sales machine.
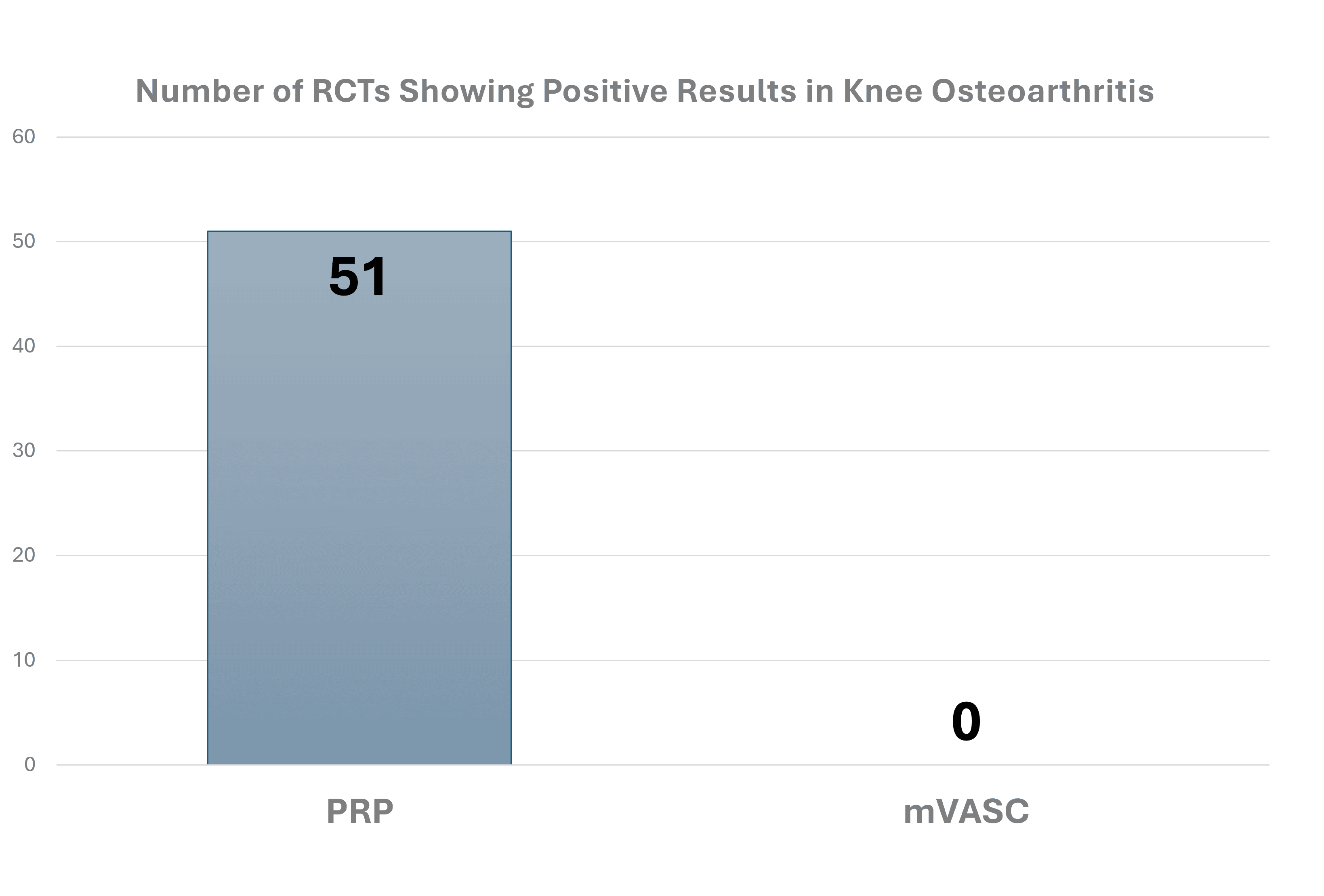
First, there are almost 150 peer-reviewed and published randomized controlled trials on platelet-rich plasma, with several dozen publications focused on treating knee arthritis. For that one diagnosis, there are thousands of patients treated in the world’s most sophisticated study designs who had success with PRP. In this case, that’s compared to a single patient in this unpublished white paper. That’s like comparing a beat up 1980’s Chevy with its engine missing to a new Ferrari for how fast both can make it around the track. See below for that comparison:
MFat?
SubQ tissue is fat. Compared to mVASC, there are about a dozen much larger and more advanced studies using your own SubQ tissue taken by liposuction and chopped up on knee arthritis, showing this treatment works well (2-6). So, if someone was really into using subQ tissue, that treatment is called micro fragmented fat, or MFat. If you’re like most Americans, you have plenty of that stuff ready to be harvested and used to treat your arthritic knee.
Safety
mVASC is a 361-registered donor tissue. Given the problems with sterility and processing found in multiple small labs selling 361 birth tissues, IMHO, any off-the-shelf 361 from a small company would earn my concern on safety. Here are multiple FDA Warning letters about those problems:
Regulatory
mVASC is a 361 donor tissue. That means that there is no FDA approval. The company only has to complete a 45-minute free online registration. Compare that to an FDA-approved drug requiring 5-7 years of clinical trials and hundreds of millions of dollars of research.
Because this is a simple 361 tissue registration, the company is VERY limited in what it can say about mVASC. Meaning the most it can say is that it is a tissue, this is where it’s from, and maybe here’s what’s in this tissue (i.e., growth factors). It can’t advertise any suggested uses for the tissue or data showing what happens when it is used in patients. Regrettably, IMHO, the company selling mVASC has already crossed that line, as we find this front and center on its website:

The FDA regulates tissues based on the claims made. If you stay on the side of a 361 registration by not making treatment claims, you can get away with the free 45-minute registration. If you make a treatment claim (IMHO, like the one above), you MUST go through full clinical trials as a 351 drug BEFORE selling your tissue. Marketing that tissue before that FDA drug approval and the years of clinical trials it requires makes the substance you’re selling a misbranded and unapproved drug. IMHO, that’s the category where mVASC lives right now.
The upshot? Given the mature data on PRP and the evolving literature on MFat, would I let someone inject ground-up cadaver subQ tissue into my knee? No way. It’s also disappointing to see yet another 361 tissue company, IMHO, playing fast and loose with treatment claims. If the company believes mVASC is the best thing since sliced bread, then great, do the clinical trials and work with the FDA to get it approved as a new drug.
______________________________________________________________
References:
(1) Dobke M, Peterson DR, Mattern RH, Arm DM, Li WW. Microvascular tissue as a platform technology to modify the local microenvironment and influence the healing cascade. Regen Med. 2020 Feb;15(2):1313-1328. doi: 10.2217/rme-2019-0139. Epub 2020 Mar 31. PMID: 32228366.
(2) Baria M, Barker T, Durgam S, Pedroza A, Flanigan D, Jia L, Kaeding C, Magnussen R. Microfragmented Adipose Tissue Is Equivalent to Platelet-Rich Plasma for Knee Osteoarthritis at 12 Months Posttreatment: A Randomized Controlled Trial. Orthop J Sports Med. 2024 Mar 18;12(3):23259671241233916. doi: 10.1177/23259671241233916. PMID: 38510323; PMCID: PMC10953019.
(3) Russo A, Cortina G, Condello V, Collarile M, Orlandi R, Gianoli R, Giuliani E, Madonna V. Autologous micro-fragmented adipose tissue injection provides significant and prolonged clinical improvement in patients with knee osteoarthritis: a case-series study. J Exp Orthop. 2023 Nov 16;10(1):116. doi: 10.1186/s40634-023-00668-y. PMID: 37968496; PMCID: PMC10651566.
(4) Yu Y, Lu Q, Li S, Liu M, Sun H, Li L, Han K, Liu P. Intra-Articular Injection of Autologous Micro-Fragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: A Prospective Interventional Study. J Pers Med. 2023 Mar 10;13(3):504. doi: 10.3390/jpm13030504. PMID: 36983686; PMCID: PMC10059754.
(5) Fan F, Grant RA, Whitehead JP, Yewlett A, F Lee PY. An observational study evaluating the efficacy of microfragmented adipose tissue in the treatment of osteoarthritis. Regen Med. 2023 Feb;18(2):113-121. doi: 10.2217/rme-2022-0110. Epub 2022 Dec 21. PMID: 36541936.
(6) Ferracini R, Alessio-Mazzola M, Sonzogni B, Stambazzi C, Ursino C, Roato I, Mussano F, Bistolfi A, Furlan S, Godio L, Alotto D, Formica M. Age and synovitis affect the results of the treatment of knee osteoarthritis with Microfragmented Autologous Fat Tissue. Knee Surg Sports Traumatol Arthrosc. 2023 Sep;31(9):3655-3664. doi: 10.1007/s00167-022-07139-4. Epub 2022 Sep 10. PMID: 36087128; PMCID: PMC10435636.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
