Necrobiology, Fake Umbilical Cord “Stem Cell” Products, and Clinical Efficacy

Credit: Shutterstock
An entire industry has been built on the idea that you can find mesenchymal stem cells (MSCs) in Wharton’s Jelly and that these plentiful stem cells can be safely used in patients without foreign tissue rejection. However, in-vitro research has shown that these cells are dead and dying. How does this impact their clinical efficacy? How does a new field of MSC research known as Necrobiology impact this industry? Let’s dig into what this all means.
Fake Umbilical Cord Stem Cell Products
The US has a weird habit of creating big industries around medical products that can be sold through armies of sales reps to gullible providers who fail to do any homework. That was how the Oxycontin debacle happened. The sales reps showed “studies” that seemed to show that Oxycontin wasn’t addictive and medical providers who should have known better bought it hook, line, and sinker.
One of the most recent examples of this phenomenon is the sale of Wharton’s Jelly (WJ) products purported to contain millions of live and functional MSCs. This is the stuff that gives umbilical cords their stiffness. While any medical researcher who has published in this space would immediately raise an eyebrow at this idea that you could thaw a frozen vial of Wharton’s Jelly in your hands and get functional stem cells, the average medical provider just doesn’t know enough to challenge the idea or understand why it has more holes than Swiss cheese.
The problem with this concept is severalfold:
- While WJ from umbilical cords saved from live births has MSCs when walked from the OB ward to the lab and placed in culture, the sourcing of these umbilical cords for products made at scale almost always kills any live cells. Why? These cords are saved by the hospital, packed, shipped to a different location, stored, processed, and bottled. By the time all of this is done, very few cells survive.
- Thawing frozen cells is something I’ve done for almost two decades now. It is NOT done by thawing frozen products in the hand, as is recommended for these products. It is actually done in a controlled rate thaw and recovery culture over days. Rapid thawing of cells as happens with these products just serves as a way to kill even more cells.
Our publication in the American Journal of Sports Medicine with CSU showed that for 6 common umbilical cord products that all claimed to be selling live and functional stem cells, none survived to be able to form cultures, the most basic definition of live and functional (1). Hence, this is why I call these WJ “stem cell” products fake.
Having said all of that, there is an emerging field of study now around dying MSCs and how they themselves could be useful in various clinical settings. So let’s first explore some basic stem cell and immune system biology and then dive into this concept. Why? Because I fear the fake umbilical cord “stem cell” industry is about to use this new data to sell these products by embracing the fact that they’re selling dead cells.
Allogeneic Hit and Run
When you transplant a donor organ into another person, that patient’s immune system immediately attacks the cells of that new organ. You can reduce this immune response by either getting a close match between donor and recipient and/or placing the patient on very powerful immune suppressant drugs. If you don’t do this, you can cause graft vs. host disease, where the recipient becomes very sick or dies due to the massive rejection response.
For MSC-based therapies, it’s long been thought that these cells were immune privileged. Immune-privileged cells don’t have markers on them that can be recognized by the body as foreign. Hence, the body never mounts an immune response attacking the foreign cells. However, recent research has questioned that idea.
A good review of this problem was published in 2014 by Harvard scientists (2). At the time, they coined the term that allogeneic MSCs worked through a “hit and run” mechanism because they could eventually be detected by the patient’s immune system and thus destroyed. This was because they weren’t actually immune privileged, but immune evasive. They postulated that if products using allogeneic MSCs were to be maximally successful, ways to continue to hide the MSCs from the host’s immune system would be required.
More Research that Allo MSCs Should be HLA Matched-“Hit and Run” vs. “Stay and Play”
I’ve blogged before that Watts et al. at Texas A&M have shown that repeated injections of allogeneic (allo) MSCs into equine joints cause the host’s immune system to be sensitized (11). They also showed that if you matched the donor MSCs to the host, they worked better because the MSCs were more likely to survive (12).
These problems don’t exist in autologous MSCs from the same patient because we know that these MSCs can “stay and play”. For example, in an intervertebral disc injection model using autologous MSCs, the cells were later detected as engrafting into the local tissue (13).
While living MSCs can evade the host’s first immune system response and get destroyed by later responses, what happens when we infuse dying and dead cells?
Treating a Patient with Dead and Dying MSCs
Almost all of the research on how MSCs work and how they may help patients has been performed using living MSCs. However, what happens if the cells are “apoptotic”? Meaning the cells are well on their way to becoming dead but aren’t dead yet.
An interesting theory that researched the idea of using dying cells for immunosuppression emerged in 2017 out of King’s College London (3). How would that work?
MSCs can cause immunosuppression in some patients, which may be helpful in targeting autoimmune diseases by reducing the body’s ability to target its own cells. The researchers found that the reason for the hit-or-miss results in allogeneic MSC clinical trials may also be related to the variable ability of patients to kill off the allo MSCs. For example, in patients who had a good immune suppression result, their bodies were better at forcing the living allo MSCs into a dying state (apoptosis). It was this act of killing, engulfing, and clearing these dying MSCs that caused the body to suppress its own immune system. So are dying MSCs as a therapy a good thing?
The Necrobiology of MSCs
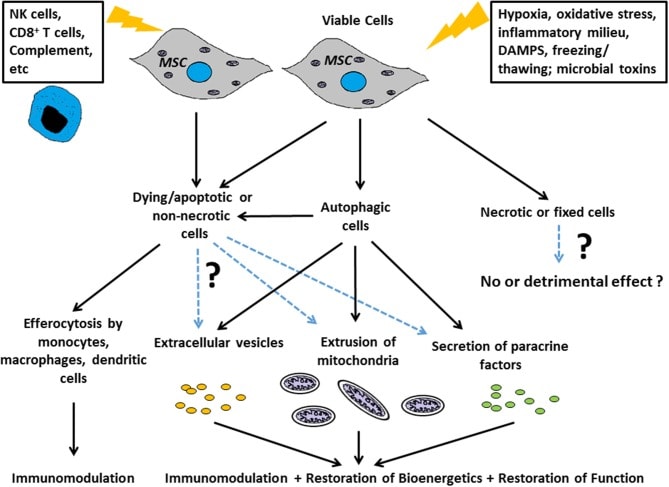
Credit: Weiss et al. The Necrobiology of Mesenchymal Stromal Cells Affects Therapeutic Efficacy. Front Immunol. 2019; 10: 1228.
There is an interesting area of MSC research emerging called “Necrobiology”. Basically, new research questions are emerging from the idea that the response to dying MSCs could prove helpful in some patients but not in others.
The illustration above is from a review paper on this topic by Weiss et al (10). This is a bit complex, but it can be summarized as follows:
- Apoptotic cells created in culture may help in specific disease states through:
- Donating their still functional mitochondria to the host’s unhealthy cells (cell batteries)
- Excreting extracellular vesicles (exosomes)
- Secreting growth factors and other helpful cytokines (paracrine factors)
While anecdotal evidence shows that apoptotic MSCs may be helpful to suppress the immune system in select patients, is there any clinical data showing that this works?
If this effect of apoptotic cells enhancing immunosuppression was a real phenomenon in living organisms, we would expect that animal models would show us that this works. However, the animal models of using dead or dying MSCs as treatment have shown that these didn’t have the same beneficial effects as live MSCs (4-7). So animal research hasn’t proven this King’s College theory.
How about human studies where we happen to have more dead versus living MSCs? A natural experiment that used MSCs to treat ARDS in humans may be helpful in illustrating this point. In a recent clinical trial on MSCs to treat severe lung disease (ARDS), the MSCs didn’t work, which made sense since 85% of them were later determined to be dead/dying (8). However, this administration was safe, likely because the 15% of cells that were healthy were able to trick the immune system of these patients into not immediately destroying the cells. That’s in contrast to another trial that used live and healthy MSCs that showed efficacy in treating this condition (9).
So What’s the Summary?
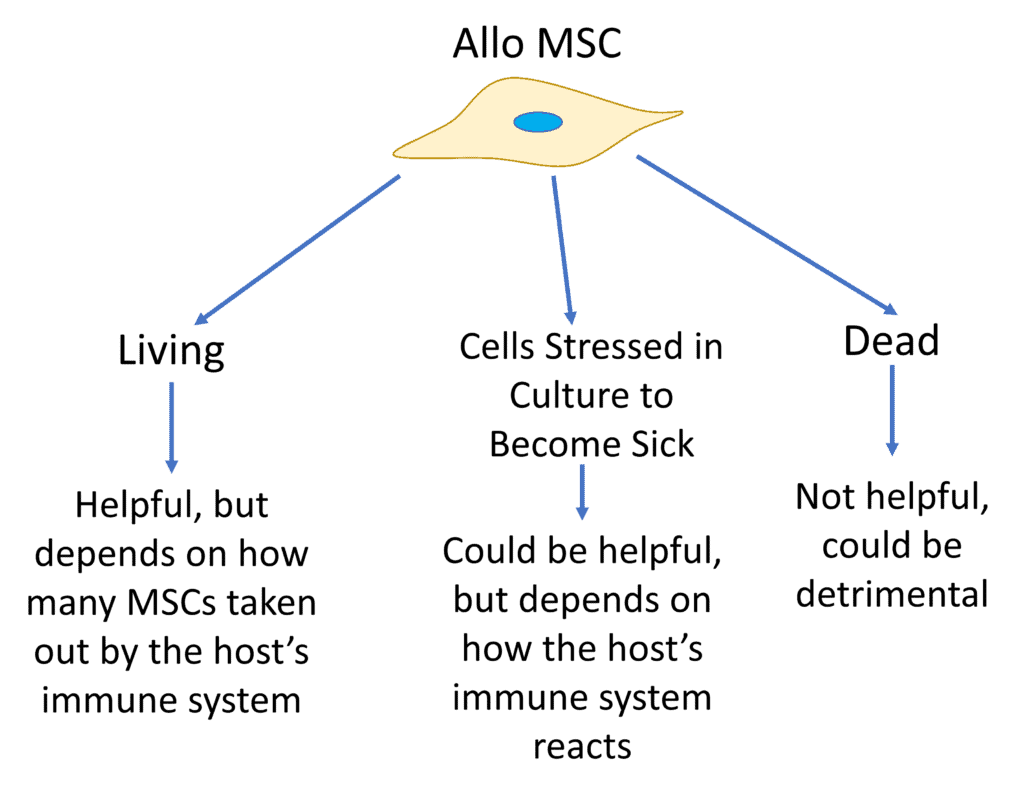
The concepts now being researched in the Necrobiology of MSCs are fascinating. However, I can already see the companies selling fake dead “stem cell” products derived from umbilical cords trying to use this to sell their products. Hence, I’ve created the diagram above that summarizes all of this research summarized by Weiss et al.
Living allo MSCs from another person could be helpful, but because they get taken out by the host’s immune system, it all depends on how close a match those cells are to the host, just like in an organ donation.
If you take healthy MSCs and stress them in culture, you can create apoptotic (sick and dying) MSCs that may also be useful in certain clinical conditions where immunosuppression is desired, but that all depends on how the patient’s body reacts. We are just beginning to understand how this works and are still far away from designing apoptotic MSC therapies.
Injecting a patient with dead MSCs (Necrotic in the illustration by Weiss et al above) is likely not a net positive. Why? This creates a rejection response because the cells aren’t able to evade the immune system and this activates different immune response pathways in dealing with dead cells that more closely resemble how the body deals with a bacterial infection.
The upshot? Could using artificially stressed and apoptotic cells be a thing in the future? Maybe, but much will depend on both honing in on how these cells are created in culture and determining which patients will benefit from this type of therapy. However, one thing is certain, you can bet that your friendly neighborhood sales rep selling WJ products may soon embrace the idea that they’re selling dead cells by trying to confuse providers that this is the same thing being discussed by Necrobiology researchers!
___________________________________________________________________
References:
(1) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413. doi: 10.1177/03635465211031275. Epub 2021 Aug 16. PMID: 34398643.
(2) Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014 Mar;32(3):252-60. doi: 10.1038/nbt.2816. Epub 2014 Feb 23. PMID: 24561556; PMCID: PMC4320647.
(3) Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017 Nov 15;9(416):eaam7828. doi: 10.1126/scitranslmed.aam7828. PMID: 29141887.
4. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. (2007) 179:1855–63.
5. Sun J, Han ZB, Liao W, Yang SG, Yang Z, Yu J, et al.. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. (2011) 27:587–96.
6. Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. (2011) 66:523–31.
7. Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al.. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. (2009) 15:42–9.
(8) Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al.. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respiratory Med. (2019) 7:154–62.
(9) Bellingan G, Jacono F, Bannard-Smith J, Brealey D, Meyer N, Thickett D, et al. Ting, primary analysis of a phase 1/2 study to assess MultiStem® cell therapy, a regenerative Advanced Therapy Medicinal Product (ATMP), in Acute Respiratory Distress Syndrome (MUST-ARDS). B14. Late Break Clin Trials. (2019) 199:A7353 10.1164/ajrccm-conference.2019.
(10) Weiss DJ, English K, Krasnodembskaya A, Isaza-Correa JM, Hawthorne IJ, Mahon BP. The Necrobiology of Mesenchymal Stromal Cells Affects Therapeutic Efficacy. Front Immunol. 2019 Jun 4;10:1228. doi: 10.3389/fimmu.2019.01228. PMID: 31214185; PMCID: PMC6557974.
(11) Joswig AJ, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R 3rd, Watts AE. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther. 2017 Feb 28;8(1):42. doi: 10.1186/s13287-017-0503-8. PMID: 28241885; PMCID: PMC5329965.
(12) Rowland AL, Miller D, Berglund A, Schnabel LV, Levine GJ, Antczak DF, Watts AE. Cross-matching of allogeneic mesenchymal stromal cells eliminates recipient immune targeting. Stem Cells Transl Med. 2021 May;10(5):694-710. doi: 10.1002/sctm.20-0435. Epub 2020 Dec 25. PMID: 33369287; PMCID: PMC8046071.
(13) Henriksson HB, Papadimitriou N, Hingert D, Baranto A, Lindahl A, Brisby H. The Traceability of Mesenchymal Stromal Cells After Injection Into Degenerated Discs in Patients with Low Back Pain. Stem Cells Dev. 2019 Sep 1;28(17):1203-1211. doi: 10.1089/scd.2019.0074

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
