New CCJ Instability RCT Study Begins…
If you follow this blog, you likely know that we have been using a new technique to treat CCJ instability that involves a novel image-guided injection rather than invasive and high-risk surgery. We have performed almost 70 procedures thus far and have seen solid results in a patient population that is otherwise very difficult to effectively manage. Now it’s time for us to try to prove beyond a doubt that this procedure is effective for CCJ instability patients. As a result, we would like to announce our IRB-approved randomized controlled trial (RCT) for CCJ instability.
What Is CCJ Instability?
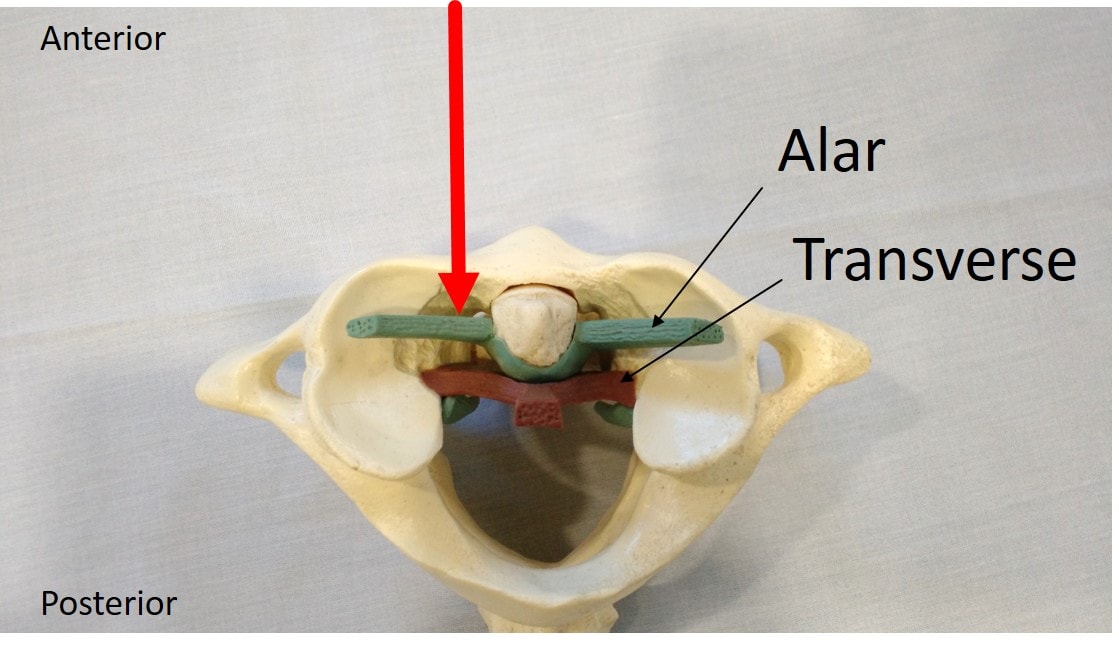
CCJ stands for craniocervical junction. This is where the head meets the neck, and it’s all held together with strong ligaments. These are the alar, transverse, and accessory ligaments. If these ligaments become injured, the upper neck joints can become unstable. This can lead to headaches, dizziness, visual problems, cognitive issues, and other symptoms. These injuries are usually caused by trauma to the head and neck or congenital conditions, like Ehlers-Danlos syndrome (EDS).
Stihii/Shutterstock
How Does the New Technique Work?
The procedure involves injecting bone marrow concentrate containing a stem cell fraction (same-day stem cell procedure) through the back of the throat (posterior oropharynx) and into the CCJ ligaments. This is performed using real-time X-ray guidance (fluoroscopy) using radiographic contrast to ensure that the injections are into the correct ligaments. This is an example of the approach:
What Has Been the Experience with the Procedure to Date?
So far, the new CCJ procedure has been used almost 70 times with no serious complications. This is important, as the only alternative is often an upper-cervical fusion surgery, which can produce significant complications. As far as outcomes, we have seen very good results in patients who we have been treating for years with many other types of injection-based and physical therapy care and who have gotten little relief. The most common reports are more stability, fewer headaches, fewer symptoms associated with the upper neck being “out,” and more ability to perform normal activities.
How Will the Study Run?
The study is a double-blinded randomized controlled trial with a six-month crossover. This means that the patient will be randomized to get either the real procedure performed or a placebo procedure (the patient is put to sleep, the back of the throat is penetrated with a needle, but the injection is not performed). In both instances, the patient will undergo a bone marrow aspiration. If the bone marrow is not used for the procedure because the patient is in the sham group, it will be donated to the research lab as a sample that can be used to perform in vitro studies. If the patient desires, the sample can also be wasted.
After the procedure, the patient is not permitted contact with the treating physician or anesthesiologist (to reduce the chances that they might figure out which group they are in). Hence, a third-party physician who doesn’t know which procedure the patient received will be assigned to the patient for post-procedure care. Any patients in the sham procedure group who have not improved by six months can cross over to get the actual procedure performed.
Who Is a Candidate for the Study?
Patients who have documented injury to the CCJ ligaments on digital motion X-ray (DMX), upper-cervical MRI (which is not a routine neck MRI image), and/or rotatory CT scan. The patient must have significant disability caused by these injuries and must have tried and failed X-ray–guided upper-neck facet injections (C0–C3 joints), nerve blocks, and upper-neck posterior ligament injections with proliferant or platelet rich plasma. Finally, the patient must have a history and symptoms consistent with CCJ instability.
Who Is Not a Candidate for the Study?
If the patient merely has headaches and/or dizziness, has had short-term relief with upper-cervical chiropractic, and has attempted no other care to remediate the problems, he or she is not a candidate for this study until advanced injection-based care has been tried and failed. If the patient is not significantly disabled by CCJ instability, this person is also not a candidate. Finally, there are a lot of diseases and problems that the patient can not have and be part of this study.
What Are the Costs?
There is no cost for the CCJ procedure. The patient or his or her insurer must bear all of the costs of the diagnostic workup, which can include the DMX, MRI, CT scan, or diagnostic/therapeutic injections. Any costs for treating complications that go beyond our Colorado clinic are the patient’s responsibility.
Where Is the Study Being Conducted?
This is a Colorado-only study for the time being. While we have approved one other Regenexx site (Belgium) to perform the paid procedure, the RCT will only be performed at Regenexx HQ in Colorado.
What’s the Difference Between This Study and Just Paying for the Treatment?
There are several differences. First, the patient will only receive bone marrow concentrate into the CCJ ligaments and not other structures that we commonly treat with the paid CCJ procedure. This includes the upper-neck facet joints and nerves. Second, the study procedure will generally not be performed by Dr. Centeno, but other clinic physicians. Finally, if there are no results, the patient is agreeing to be locked into the observation period of the study for a minimum of six months, where no other care (outside of that approved by the study authors) can be delivered.
The upshot? We’re excited to get started on this first-of-its-kind randomized controlled trial. We feel that we have gotten expert enough at this procedure and that it seems to be helping enough patients that it’s time that we start to prove beyond a reasonable doubt that the procedure works in a select patient population. If you have an interest in being a study patient, contact our research coordinator, Ehren at [email protected].

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.