Platinum Biologics: Beeben 2.0

Remember, back in the 70s and 80s, there was a phrase after the movie Jaws 2 came out, “Just when you thought it was safe to go back in the water…” I feel that way regarding the last few years’ umbilical cord “stem cell” scams. The FDA and CMS came down hard on the bad actors in that space, and you would think that here in mid-2024, it would be safe to go back in the water, meaning these scams would have evaporated. However, you would be wrong.
I’m Getting Tired of Calling Out the Bad Actors
This has been a great few years for autologous orthobiologics. RCT after RCT has been published, showing that PRP and bone marrow concentrate work. The fake PRP studies have been exposed, and because of the great results, orthobiologics are spreading like wildfire throughout the physician community. In addition, while there are still plenty of scammers, the big companies supplying the scam clinics have seemingly dried up after multiple FDA warning letters and CMS actions on fraudulent Medicare billing. Hence, you’ve seen fewer blogs from me about the stem cell Wild West. In addition, I have to admit that there is inevitable fatigue on my part as I’m getting tired of calling out the bad actors. After all, if a consumer is not sophisticated enough to know that a fake IV stem cell treatment isn’t going to do anything to treat their knee or neck, as PT Barnum once said, “There’s a sucker born every minute.”
A Flyer Left for a Colleague
I get emails from outraged physicians who know they’re getting scammed, thanks largely to my hundreds of published blogs on these subjects. I recently got an email from a physician who a company contacted out of Florida called Fruition Biologics. The sales rep was Chris Raney, so I called Chris, an ex-insurance salesman. I have to say that I felt sorry for him, as IMHO he had no idea what he was selling or the laws about selling 361 registered birth tissues for orthopedic use.
What’s the problem? He sent the following to my colleague:

The flyer compares a Wharton’s Jelly product called “Nano PRP Jelly,” sold by Platinum Biologics, to PRP. Interestingly, it has a disclaimer that says: “All Platinum Biologics products are intended for cosmetic, research and/or homologous use.” So, let’s see if this product can actually fit into any of those categories.
First, what is a cosmetic?
“Federal law defines “cosmetic” as “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and (2) articles intended for use as a component of any such articles; except that such term shall not include soap.” 21 USC 321(I).
We now know this product’s objective intent was not for topical cosmetic use as defined above, so this part of the disclaimer is untrue. Why? The flyer was sent to an interventional spine physician and relayed, “Administer practically anywhere you have previously used PRP through any 25-32 gauge needle.” This physician used no topical cosmetics in his practice and would only be interested in this product if he could inject it into a peripheral joint or spine.
Next, we are told that this product is for “research use.” That’s an interesting disclaimer, as the FDA has stated in multiple Warning and Untitled Letters that research in orthopedics with Wharton’s Jelly requires a drug IND and then subsequently, upon eventual FDA approval, a Biologics License [42 U.S.C. 262(a)] (1-10). Hence, selling this to my colleague under the guise of research would require his office to have an FDA IND to perform that research. This would have cost my colleague millions of dollars, causing him to hire a third party to implement an IND. Even if he attempted an SPEA, he would need to hire expensive outside experts to put this single-use, expanded access form together, and that would only apply if this product already had a drug IND listing showing safety trials. Hence, this part of the disclaimer fails as well.
Finally, the disclaimer states that this Wharton’s Jelly product is for homologous use, but multiple FDA Warning and Untitled Letters have made it clear that when these products are used to treat orthopedic or pain conditions, they are non-homologous based on 21 CFR 1271.10(a)(2) (1-10). For example, the FDA stated in its Warning Letter to Regenative Labs (that company will become important later in this tale):
“Regenative Labs does not qualify for any exception in 21 CFR 1271.15, and your products fail to meet all the criteria in 21 CFR 1271.10(a). Specifically, your products fail to meet the criterion in 21 CFR 1271.10(a)(2) that the HCT/Ps be “intended for homologous use only, as reflected by the labeling, advertising, or other indications of the manufacturer’s objective intent.” Your products are not intended to perform the same basic function or functions of the umbilical cord in the recipient as in the donor, such as serving as a conduit. Using your products to treat orthopedic diseases or conditions, for example, is not homologous use as defined in 21 CFR 1271.3(c).”
Hence, this part of the disclaimer blows up as well.
So, is Nano PRP Jelly (the Platinum Biologics Wharton’s Jelly product) a cosmetic? No. Is Nano PRP Jelly a 361-tissue product? No, it fails the tests (found in 21 CFR 1271.10(a)), so it can’t be classified that way. Is Nano PRP Jelly as sold in this marketing email and based on the objective intent of the seller a 351 drug? Yes. The product is being sold to treat MSK conditions, it is processed to destroy its structural properties so it can be injected, it is not being used in a homologous fashion, and finally, the objective intent is not to sell it to a cosmetic dermatologist who might use this on a patient’s skin in a topical fashion, but for injection into the MSK system. Since Platinum Biologics has no 351 drug approval or IND/Biologics License Application, IMHO Nano PRP Jelly is an unapproved and misbranded drug product.
Beeben 2.0
So, who is behind Platinum Biologics? None other than Beeban Russell, former VP of sales for Regenative Labs (the company that received the above warning letter):

If you recall, this sales rep and later VP of Sales for Regenative Labs had a single year of high school. That’s important here because, IMHO, he and others at Regenative were responsible for causing physicians to bill Medicare for orthopedic uses of Regenative Wharton’s Jelly products. That whole Medicare scam involved Q-codes and billing Medicare for non-covered procedures. Based on what I have ascertained, the later clawback of those payments by CMS was one of the largest refunds from individual doctor’s offices in Medicare history.
The New WJ Sales Dodges
We’ve heard the homologous use dodge before. For example, “My Wharton’s Jelly product is being used as a cushion for the knee cartilage; hence, it’s homologous use as that’s what this substance does in the umbilical cord”. As you can see above, the FDA wasn’t buying that one. However, what’s new here are the “Research Use Only” and “Cosmetic” dodges.

Basically, it is prohibited to call your product “Research Use Only” (RUO) if you sell it to doctors or patients who use it to treat medical problems.
The newest dodge for WJ products I hadn’t heard before was “Cosmetic Use.” I spoke to Mr. Russell on the phone because he was upset that I had the audacity to call his sales rep. He claimed that these WJ products were being sold for topical use; hence, he wasn’t even required to register these products as a 361 biologic. In fact, I couldn’t find his company as a manufacturer or vendor in the FDA Tissue Establishment Registration system:

IMHO that’s pretty crazy. Even though, based on its marketing, this product is a misbranded and unapproved 351 drug product, the FDA has no way to find this tissue in its own 361 registration system because it’s not even registered!
PRP versus Wharton’s Jelly
In my experience, a common sales pitch that Beeben and many WJ sales reps use here is that the products are “better” than PRP (see sales pitch above for Nano PRP Jelly). However, if we make that comparison based on the number of positive RCTs for something like knee arthritis, this is what that looks like:
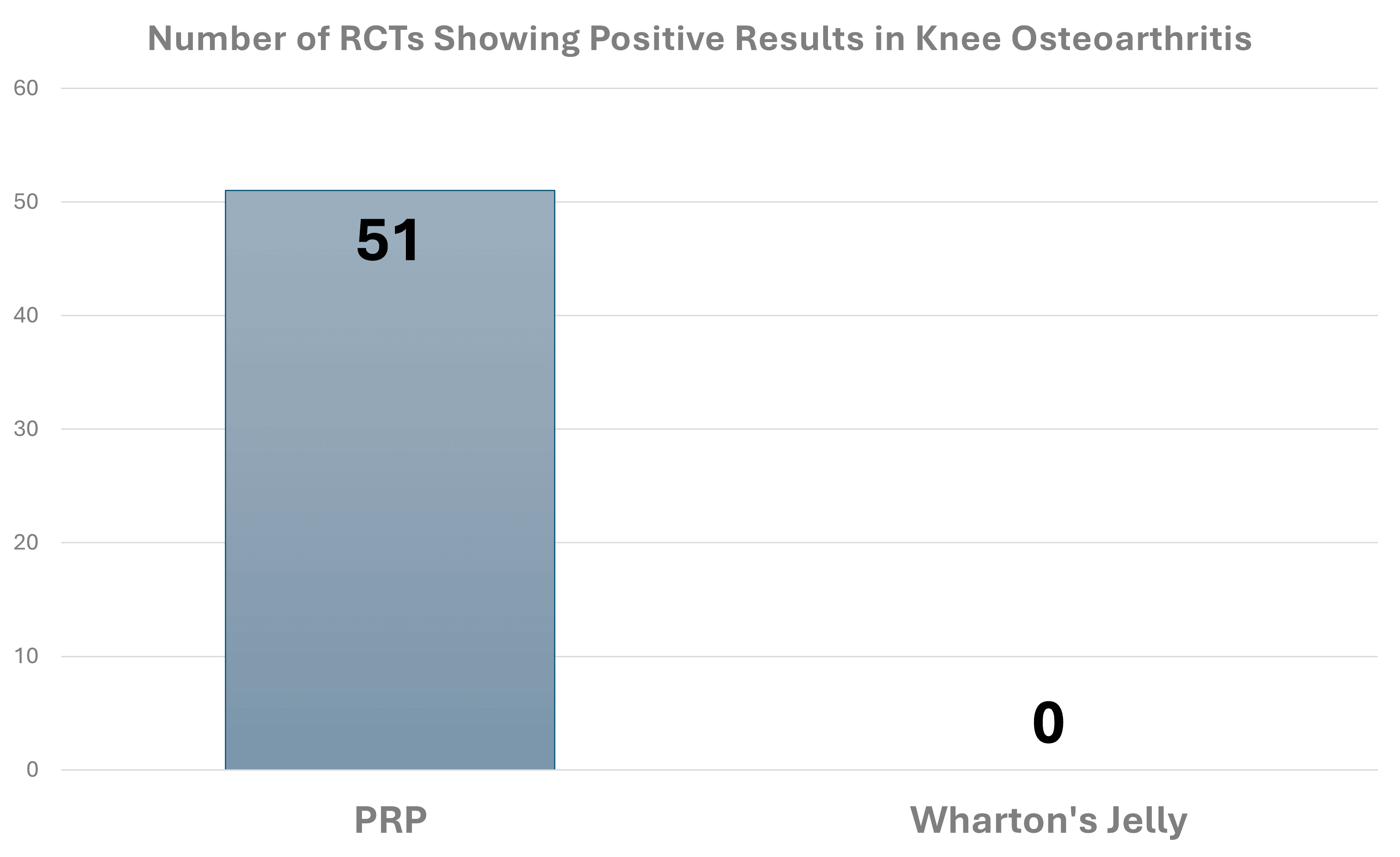
There is no comparison. IMHO, any physician right now would be a fool to use a WJ product based on the existing science.
The upshot? It’s increasingly clear that it’s not safe to get back in the water. Beeben 2.0 is just the tip of the iceberg of old and new Wharton’s Jelly sellers trying to push product once again. It will be interesting to see what the FDA’s next move is, as in my experience, just as they close down one company, it’s so easy to start a new venture that the next one pops up! I think it’s time for the FDA to end its tissue registration system and begin declaring any donor tissue a drug or creating a 510K clearance system with a predicate that increases the bar for sellers of these tissues. IMHO, only then will this madness end.
__________________________________________
References:
(1) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/invitrx-therapeutics-inc-630712-11092022
(2) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/signature-biologics-llc-631039-09182023
(3) https://www.fda.gov/news-events/press-announcements/fda-sends-warning-companies-offering-unapproved-umbilical-cord-blood-products-may-put-patients-risk
(4) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/neobiosis-llc-662985-06052024
(5) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/renatilabs-inc-646353-06012023
(6) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/stratus-biosystems-llc-dba-cellgenuity-regenerative-science-631303-06052023
(7) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/smart-surgical-inc-dba-burst-biologics-614361-02022022
(8) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/genetech-inc-561808-11292018
(9) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/vitti-labs-llc-627699-07282022
(10) https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/row1-inc-dba-regenative-labs-638823-06212023

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
