Regen America Review: The Wild West of Stem Cells
The stem cell wild west gets worse every month. This past year, we’ve seen the entry of chiropractic groups offering stem cell therapies, representing a paradigm shift in the treatment of orthopedic injuries. As a result, the hyperbole factor and targeting of the elderly has increased. Today I’d like to perform a review on Regen America, one such chiropractic stem cell group. As always, I’d like to review this group using the same methods you would use to ascertain whether any orthopedic stem cell treatment is likely “the real deal.”
The Great Amniotic/Placental Tissue Deception
If you read this blog, you know that the biggest scam going today in regenerative medicine is FDA-registered birth-tissue vendors claiming that their products are loaded with live and functional stem cells. Not only is this not the case based on independent testing, but also these claims are illegal from an FDA standpoint. So when doctors (or chiropractors) repeat this claim, it’s a problem.
So with all of that in mind, let’s take a look at Regen America.
The first thing that hits you is a reservation form that allows you to reserve a spot at a local seminar. There’s also a video hosted by Forbes Riley (I didn’t know who this was). Let’s delve into the content of that video.
Interviewing a Chiropractor
First up in the video is an interview of Jill Howe, DC. Before we go any further, when someone is presented as an expert in any medical field, there are some things you can expect to see if they actually are an expert, and the biggest is original scientific publications on that topic. Hence, I ran a quick search on the US National Library of Medicine and found no publications on stem cells authored by Jill Howe, DC.
Now let’s delve into what she’s saying:
“There are different places that you can get stem cells, but the place that we get them is from Mom’s…”
Dr. Howe is describing the use of amniotic/placental tissue products to treat orthopedic conditions.
REALITY CHECK: THERE IS NO AMNIOTIC OR PLACENTAL PRODUCT ON THE MARKET IN WHICH WE OR OTHERS HAVE IDENTIFIED VIABLE AND FUNCTIONAL STEM CELLS. IN ADDITION, A CLAIM OF LIVE CELLS MAKES THESE PRODUCTS UNAPPROVED, ADULTERATED, AND MISBRANDED PRESCRIPTION DRUGS.
The claim that Regen America is offering a live and viable stem cell therapy that is derived from live births is not in keeping with the evidence that we have collected after testing these products, which show them to be dead tissue. In addition, the IOF tested these products and also found the same:
More importantly, a manufacturer of such tissue merely making a claim that the tissue has live stem cells is a clear violation of FDA regulations. To understand why, see the video below:
“The number of stem cells in your body declines with age.” While true, this is, of course, not the whole story. What’s not said is whether this decline reduces the effectiveness of stem cell therapies derived from the patient? While Regen America has no clinical research listed on its website, our registry-based, published research in knee osteoarthritis has shown no age-related effects. Meaning being older doesn’t reduce your chances of responding to a treatment using your own stem cells. In addition, Regen America isn’t using a live stem cell product, so the discussion about stem cell number is moot.
“This particular type of therapy, when it’s injected, will go in and regenerate that tissue that you’re missing. You can get new cartilage, you can get new knees, new shoulders, new nerves” Of all of the most deceptive things these new chiropractic clinics using various types of stem cell therapies put out there, it’s pitching to older patients with severe arthritis that “stem cell therapy” will grow you a new joint. So let me make this VERY CLEAR. As the first physician on earth to inject stem cells into a human osteoarthritic knee joint, with many publications under my belt, and having authored many book chapters and taken hundreds or pre and post MRIs after having used many different types of stem cells, there is no stem cell therapy that will grow a patient a new joint once a patient has severe arthritis. Given that Regen America isn’t even using a stem cell therapy at all, but a placental or amniotic growth-factor shot, it’s upsetting to hear Jill Howe talk about stem cells and then tell prospective patients that she can grow them a new joint.
“There have been over 300,000 treatments done with this particular product with not one reaction.” This is a deceptive statement on multiple levels. First, “this particular product” refers to the amniotic/placental tissue they’re using. There is no such product that has tracked 300,000 treatments. In addition, to make this statement, you would need an advanced registry following 300,000 patients constantly pinging patients about side effects. This doesn’t exist. So this statement isn’t supported by any credible and published scientific analysis of the carefully tracked side effects of amniotic and placental tissues.
Second, this is deceptive because these products do routinely produce extreme inflammatory responses. In fact, we have physicians who refuse to use them because of that inflammatory reaction. In addition, there is no medical therapy ever devised that has no side effects.
Finally, any tissue from a donor can cause infection in a patient, regardless of donor testing. I’ve already blogged about a patient who was a family physician who received a donor fibrin-glue product, and despite FDA-mandated screening tests, still contracted hepatitis C. This false-negative rate for diseases like HIV is discussed in this CDC publication (ref).
Interviewing a PhD
The interviewer, Ms. Riley, then goes on to interview a PhD who is presumably from the University of Florida, Mark Moore. In fact, he was never a tenure-track professor at the University of Florida, but that’s where he did his post-doctoral research/training being sponsored by a company called BioD. Prior to that, he worked for BioD. The IOF tested the BioD amniotic fluid product after the company claimed live cells, but no live cells were found. He’s now at the University of Oklahoma. His bench research has focused on using the growth factors in placental tissue or characterizing BioD’s products, with nothing published in the orthopedics space.
A web search shows that he is now at the University of Oklahoma. I was a bit confused at his title, which is an Assistant Professor at Practice? Turns out that this is another industry-focused job without a tenure track leading to a full professorship. So let’s listen to what Mark has to say in the video:
Riley: “What has the research told you about regenerating the joints in the knees, hip, and the shoulders?”
Dr. Moore: “The studies have shown that they have the ability to regenerate tissue, reduce pain and inflammation within the joints of hips and elbows, etc…”
Riley: “So how much testing is done to make sure that it’s safe?”
Dr. Moore: “They have decades-old research to show that they’re safe, effective, for healing all sorts of tissues…”
REALITY CHECK: THERE IS NO PUBLISHED RESEARCH ON PATIENTS WITH KNEE, HIP, SHOULDER, OR ELBOW ARTHRITIS WHERE AMNIOTIC AND/OR PLACENTAL TISSUES HAVE BEEN SHOWN TO BE “SAFE AND EFFECTIVE.”
What’s a bit bizarre about this exchange is that a current university-affiliated PhD is clearly exaggerating the status of the clinical research. It would be interesting to get the take of the University of Oklahoma on this video.
What’s Not Found Is as Important as What Is Found
There are certain things you should look for in any clinic advertising stem cell therapy to treat pain or arthritis. These aren’t found on the Regen America site:
- Published research on stem cell use in orthopedics that uses the therapy that is being advertised in real patients. In this case, where are the research studies published by Regen America on knee, hip, and shoulder arthritis?
- A patient registry that tracks all treated patients. This is an ethical requirement IMHO. This means that there is a small army of people whose job it is to track patients for complications and outcomes after the therapy.
- Qualified medical specialists delivering the care. In my book, this would be an MD or DO physician who has taken and passed the IOF courses (or similar) for regenerative injection-based orthopedics (interventional orthopedics).
This is a very short list.
Now, here’s another reason not to get stem cell procedures performed at your local chiropractor’s office:
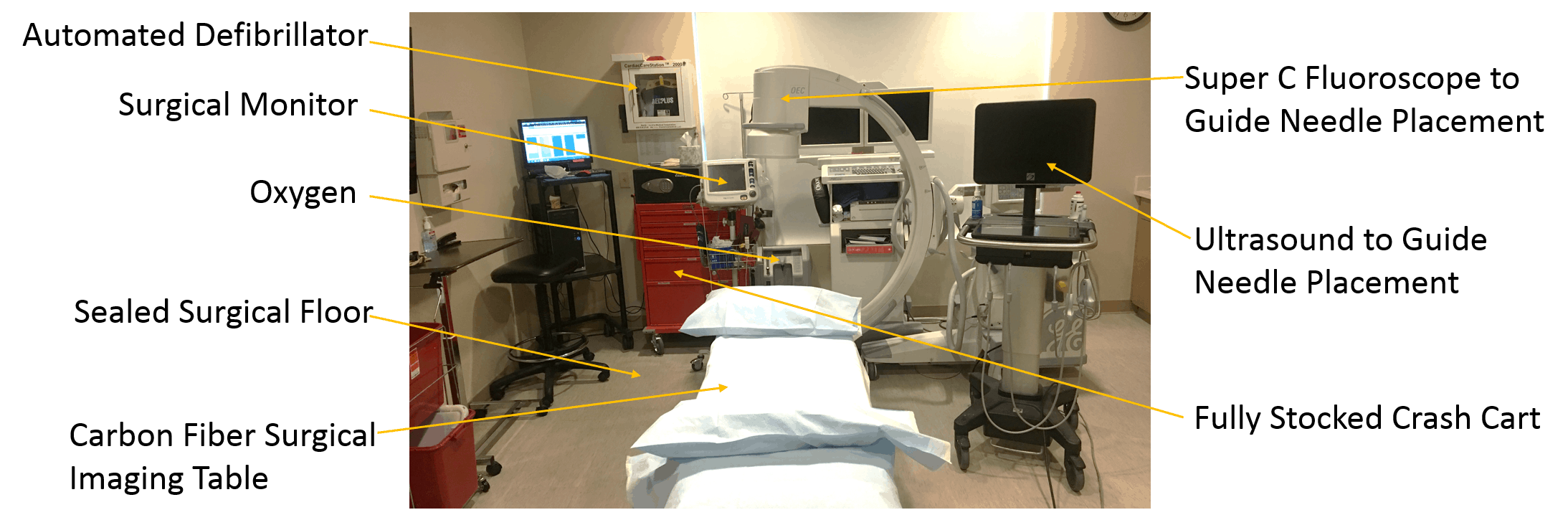
The upshot? So far, from what I can see, Regen America is one of many chiropractic amniotic/placental tissue networks making wildly exaggerated claims about the efficacy of “stem cells” while using dead nonviable tissue that doesn’t actually contain functional stem cells. What’s really disturbing is that the target appears to be elderly patients who are the most likely to be misled into believing that this is a miracle cure that will regrow them a new joint.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.