The Regen Med Research Has a Dirty Little Rat Secret
One of the more interesting things about regenerative medicine research is that all too often, the results that look great in rats fail to be replicated in humans. In fact, as I’ll argue below, this has caused a big problem for the field all based on the technical difficulties of rat research. Meaning it’s because of beady-eyed rats that the field has been set back decades. Let me explain.
A Human vs. Rat BMA
Most physicians with regen med experience that review the literature or attend conferences don’t realize that pretty much all small animal models involve pooled cells. This is a HUGE problem that’s almost never discussed. Why? Let’s dig in.
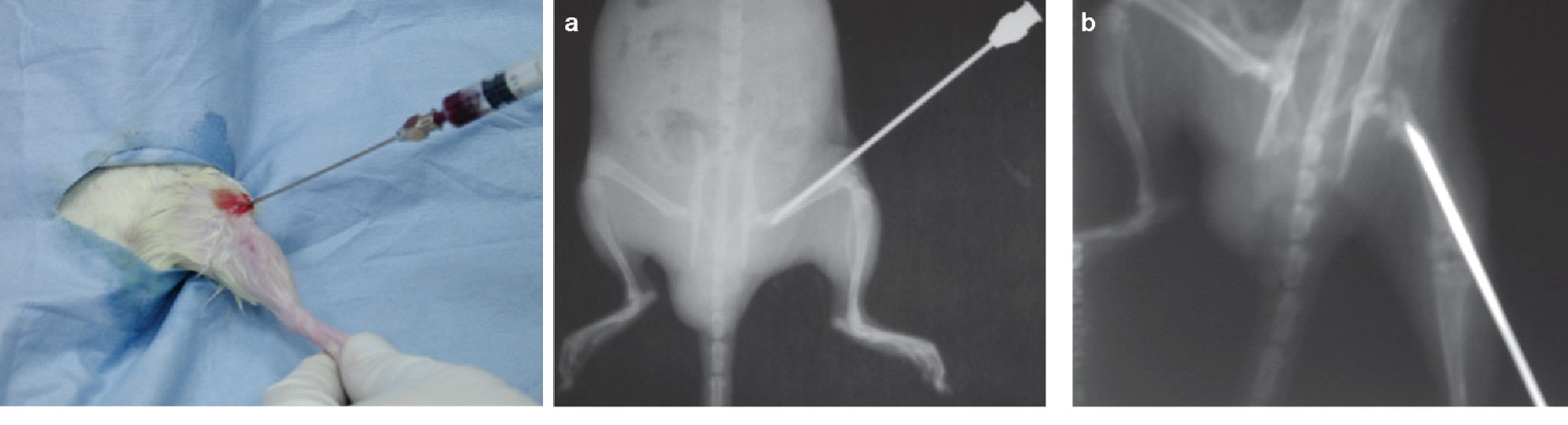
A bone marrow aspirate is how a doctor takes the liquid portion of the stem cell rich bone marrow through a thick needle called a trocar. The same procedure is used for animal stem cell research. However, getting bone marrow out of a rat is VERY hard.
As physicians, we take for granted that we use a relatively tiny needle compared to the size of the human pelvis when we perform a bone marrow aspirate procedure. Now take a look at the rat BMA above. The thickness of the needle is the same size or bigger than the width of the rat pelvis or long bones! Now think about getting enough for autologous stem cell therapy. Given that sticking this huge instrument in tiny bones usually kills or maims the rats, that’s pretty tough to do. These practical problems have wreaked havoc for regen med research and this is a dirty little secret nobody wants you to notice.
The Pooled Cell Model
Given the limitations of human hands, rat bones, and easily available needles big enough to allow bone marrow aspirate to flow, you can’t get much bone marrow out of a single rat. Hence, you need to pool the bone marrow of many rats to get enough to work with and culture expand. Hence, almost all small animal models involve a pooled cell line. Meaning you have the cells from many rats or mice or rabbits that you then grow in culture and use experimentally in other animals to try to heal damage.
Why Pooled Rat Cells Cause Problems for Human Translation
We used to believe that mesenchymal stem cells were immune privileged. This meant that you could take stem cells from any donor and place them in any recipient without any chance that the recipient would reject the cells. However, it turns out that this is only about half true.
We now know that while someone else’s stem cells placed in your body will be immuno-evasive, they are not immune privileged (1). Meaning that they can only work through a paracrine hit and run mechanism because they eventually get taken out by the host’s immune system. Hence, they get about a month or so to excrete cytokines, growth factors, exosomes, etc… They do this so that they can have other cells do the repair work.
Ashlee Watts, a researcher at Texas A and M has also clearly shown that the closer the cells are HLA matched to the recipient, the better they work (2). Why? They likely last longer before being gobbled up by the host’s immune system. Meaning if the stem cells used for treatment have surface molecules that match or closely match the treated animal or patient, they will survive (i.e. a close HLA match).
Now let’s think about pooled cells from many animals. Despite these rats being bred to be as close as possible, they’re also bred to maintain genetic differences. This helps animal research apply better to us genetically diverse humans. Hence, their cells have many different HLA phenotypes and pooled stem cell experiments have lots of cells with many different HLA types.
HLA Matches and Immune Responses
If you take the cells from any given animal and place them into another animal, as Dr. Watts did above, you have a small chance that the two animals are a close HLA match. If that happens, the transplanted cells last longer and work better. However, if the stem cells aren’t a good match you have problems as the cells don’t work as well or at all. Now consider taking stem cells from many animals. The likelihood that you have a random or close match goes way up. Hence, using pooled stem cells in an animal model is much more likely to be effective than a single donor model. However, most of the cells get taken out. Given that almost all of our basic science research is based on a pooled stem cell model, this could explain why many animal models don’t translate into human clinical trials.
Single Donor Human Stem Cell Products
Now that we know that the animal research results on which many companies have based donor stem cell products is flawed, let’s consider those products. First, for many reasons, they are not pooled stem cell products from many donors, but instead from a single donor. Hence, they have the same problem as Dr. Watts’s study, if you happen to get a close match with the recipient, the cells will work better, if not, then they won’t. In fact, this likely explains why many off the shelf stem cell products have failed to work as well in human trials as they did in rats. In fact, it’s my guess that this is what launched the entire push in the literature today to consider mesenchymal stem cells (MSCs) renamed as medicinal signaling cells. Let’s explore that a bit.
What’s in a Name? Medicinal Signaling Cells
A big push is afoot to rename mesenchymal stem cells as medicinal signaling cells. This is based on the research showing that these cells don’t differentiate as once thought, but instead only stick around long enough to excrete signals to other cells. However, now you know that all of that the basic animal research is flawed by its very nature. This is because it was done with pooled cells, many of which are being taken out by the host’s immune system, but some could end up being a close match and survive. However, all human clinical trials are performed with the stem cells of a single donor. Hence the stem cells used are less likely to engraft because they’re highly likely to be taken out by the host’s immune system. Hence, what better way to explain away a self-created problem than to rename MSCs?
Autologous Stem Cells Stay and Play
Allogeneic stem cells (someone else’s cells) get gobbled up by the host’s immune system, so whatever effect they have is “hit and run”. However, no such rejection issues exist with autologous cells. So do they act differently? Yep.
Take for example this study that transplanted autologous mesenchymal stem cells from bone marrow concentrate into the degenerated discs of several patients (3). The cells not only survived but they differentiated and engrafted into the discs. Or this study of autologous equine MSCs placed into tendons, which showed that unlike allo MSCs that got taken out after a month, these were still around by the end of the experiment at 9 weeks (4). So autologous MSCs stay and differentiate. This makes autologous stem cell therapy a completely different “animal” than pooled or single donor allogeneic therapies. It might as well be a called something different. So if the allo guys are going with medicinal signaling cells, I’ll stick with mesenchymal stem cells for autologous therapies.
Why Haven’t You Heard that Much About this So Far?
This idea that we have screwed up the animal research because it’s hard for grad students to get the bone marrow from rats is not something you hear about. In addition, outside of an Ashlee Watts lecture you barely hear about the fact that the single donor stuff winding its way through FDA trials is a very flawed therapy idea. Why? Billions have been spent and bet on the idea that we can use allo stem cells that are mass manufactured in vials. Nobody wants to hear anything else at this point.
The upshot? Now you know that our basic science research in mesenchymal stem cells and regen med has a dirty little rat secret. Since grad students have a hard time getting enough bone marrow for an autologous experiment without killing the rat, we have pooled MSC data that is seriously flawed as a translational research model. This has caused our single donor stem cell product pipeline to be based on a broken therapeutic concept. Hence, expect more cell therapy trials to fail or underdeliver until we get a universal donor scheme figured out. In the meantime, it’s an autologous stem cell world baby!
________________________________
References:
(1) Ankrum, J., Ong, J. & Karp, J. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32, 252–260 (2014). https://doi.org/10.1038/nbt.2816
(2) Rowland, A.L., Xu, J.J., Joswig, A.J. et al. In vitro MSC function is related to clinical reaction in vivo. Stem Cell Res Ther 9, 295 (2018). https://doi.org/10.1186/s13287-018-1037-4
(3) Henriksson HB, Papadimitriou N, Hingert D, Baranto A, Lindahl A, Brisby H. The Traceability of Mesenchymal Stromal Cells After Injection Into Degenerated Discs in Patients with Low Back Pain. Stem Cells Dev. 2019 Sep 1;28(17):1203-1211. doi: 10.1089/scd.2019.0074.
(4) Geburek F, Mundle K, Conrad S, et al. Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions–a pilot study. Stem Cell Res Ther. 2016;7:21. Published 2016 Feb 1. doi: 10.1186/s13287-016-0281-8

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
