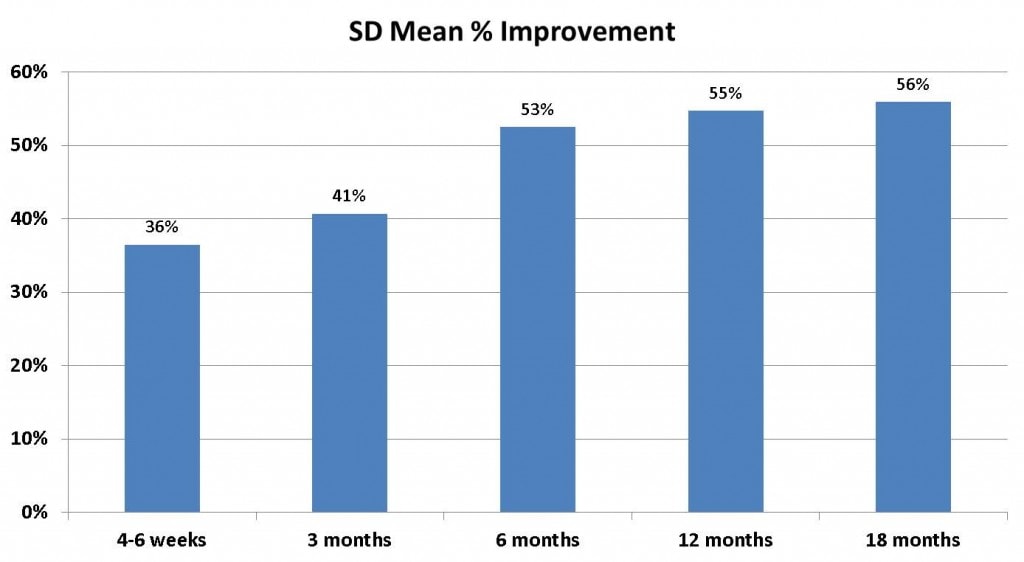
Regenexx-SD Data
First, while I had planned to release the new book today, some hang ups on the cover art has made that more like likely to happen tomorrow. Instead, I’d thought I’d share some very preliminary Regenexx-SD data on knee arthritis patients I just got yesterday. We now spend massive amounts of money on data collection in our patients. This is about 200K USD annually plus we’re doubling down and re-engineering and improving our custom software for data collection this year, likely a significant additional 6 figure expense. Why spend about 1/3 of a million dollars this year on registry based data collection when other physicians offering these therapies don’t do this and many companies selling FDA approved bedside devices to obtain a stem cell fraction from marrow also don’t collect and publish data? We do this because it’s the right thing to do with new technology. First, the data above is a snapshot of all tracked Regenexx-SD knee arthritis patients for the last 18 months-2 years. As our research director continues to refine this information over the next 1-2 weeks, you’ll see more information and he will slice and dice this in many different ways. This graph represents 184 patients (134 males and 50 females). Also realize you’re looking at all patients where we could wrestle a response, so this registry approach isn’t equivalent to a randomized controlled trial. However, it does show average responses per time point for a group of patients who were mostly knee replacement candidates. So at 18 months, 56% means that on average, in that group of patients who were 18 months out, the average improvement was 56%. This means some patients might have only had a 30% improvement and some might have had a 70% improvement. It doesn’t mean that stem cells cure everyone, which is something we’ve been adamant about from the beginning. It also doesn’t mean that a patient at 18 months can expect to get 56% improvement. I’ll keep the -SD and -AD data reports coming over the next two weeks as I see interesting things to talk about. In addition, once it’s all been put out there, we’ll do what we usually do, submit it for publication. You should also know that I authorized two Randomized Controlled Trials of Regenexx-SD this week-one on up to 1 cm complete rotator cuff tears and one on knee meniscus tears-both free to patients who qualify at three different Regenexx network sites. As more info becomes available on those studies and as we begin recruitment, I will post more details. The upshot? We take our data very seriously.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.