Stem Cell Centers Review: Not What it Seems
As you know, I review various clinics that pop up on my radar. It’s usually pretty easy for me to spot the good from the bad ones. So what did I think about this one? Read on for my Stem Cell Centers review.
Selecting a Stem Cell Clinic
I’ve been publishing research on stem cell use in orthopedics and treating patients since 2005. Hence, if you had me looking over your shoulder when performing research on the web, I could very quickly tell you what and who to avoid. Obviously, I can’t do that for everyone, but I did write a small book that helps you figure out where to find high-quality stem cell care for orthopedic injuries:
Stem Cell Centers Review
This clinic came on my radar this week when I saw a Facebook ad like the one shown here. Looks like this group has clinics in northern Colorado, Broomfield, Denver, Aspen, Colorado Springs, Kansas City, Oklahoma City, Dalla/Fort Worth, and San Antonio. This Facebook ad says that they offer “an injectable solution that oftentimes leaves the patient feeling relief after only ONE treatment.” Hmmm… So what is Stem Cell Centers injecting? This what the web-site says:
“While ethical debates have arisen about embryonic stem cell therapy, most everyone agrees that the use of other Stem Cell Therapy raises no ethical or moral questions. Cord-Blood Stem Cell therapy also has an advantage over other methods because these stem cells carry no threat of patient rejection. The stem cells are “neutral” cells which have no DNA in them making everyone a match.”
It says here that their injectable solution is made from umbilical cord blood. Next, I called the free consultation phone number and I spoke to a representative. She told me that they don’t use umbilical cord blood, but instead amniotic fluid. They get that product from Utah Cord Bank. The rep had no idea which of the three products made by Utah Cord bank that the clinic used. In fact, she seemed highly confused as to the differences between umbilical cord blood and amniotic fluid. Meaning, in her mind they seemed to be the same thing. No matter that one surrounds the baby (amniotic fluid) and the other is taken out of the blood vessels in the umbilical cord. Let’s dive into what we know about umbilical cord products from Utah Cord Bank.
Testing Umbilical Cord Products for Live Stem Cells
We have hundreds of seminars a month going on throughout the US that state that clinics are using amniotic and umbilical cord products that contain millions of live and functional stem cells. Regrettably, this is a fiction not supported by several scientific studies that have tested these products (1-3). Meaning, actual research studies published and presented at scientific conferences show that all of these birth products used by clinics have NO living stem cells. In fact, a group of academic physicians felt so strongly about the problem of clinics like these claiming that amniotic and umbilical cord products had stem cells, that they produced a position paper on the topic.
In this case, both our lab and the Colorado State University lab have tested the StemVive product from Utah Cord Bank and found that it contains zero living mesenchymal stem cells, despite what is promoted on the Utah Cord Bank website. Want to see visual proof of that? Just look at the culture plates below from our lab. Each purple dot is a stem cell colony (called a CFU-f). On the left, you see StemVive, which shows no purple dots, so no stem cells. On the right, you see bone marrow from a 51-year-old man, which shows a significant number of stem cells.
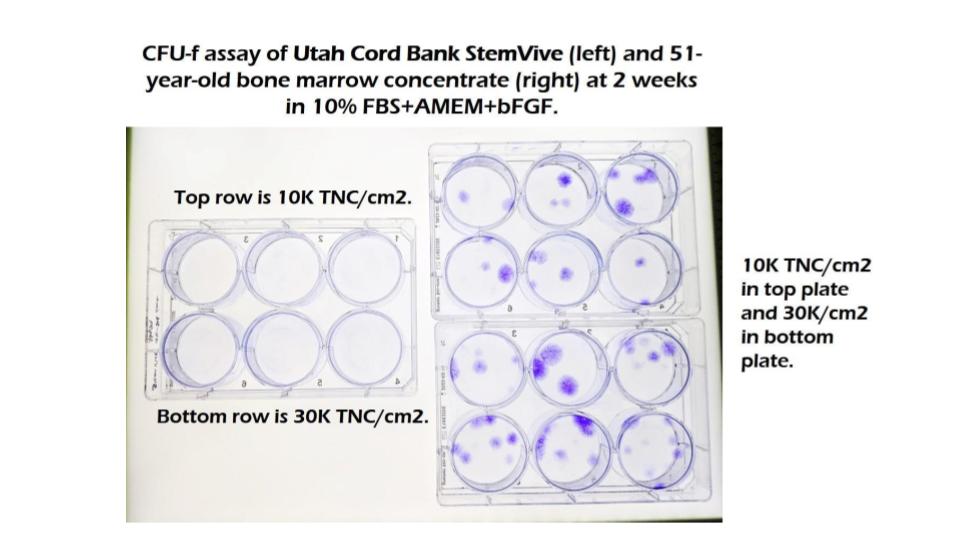
Hence, we have Stem Cell Centers claiming that they are injecting stem cells, but independent university tests of a product from their birth tissue supplier shows that it has no live stem cells. Meaning, based on the research to date, what you’re getting at Stem Cell Centers is not a stem cell injection.
More Problems: Not Understanding Basic Biology
As a medical doctor, one part of the statement by Stem Cell Centers above really bothered me. That was this:
“Cord-Blood Stem Cell therapy also has an advantage over other methods because these stem cells carry no threat of patient rejection. The stem cells are “neutral” cells which have no DNA in them making everyone a match.”
What Stem Cell Centers is referring to here is the fact that when cells or organs from another person are implanted into your body, your body can reject that tissue. That’s because there are markers on the surface of those cells that your body reads to see if they are your cells or a foreign invader (called HLA markers or the MHC proteins) (7). Think of this as a bar code system. Your white blood cells have a scanner that checks the bar code on all cells they pass. If they’re not yours then they attack that cell in a process called rejection.
So if we take the first statement, that cord-blood stem cell therapy carries no risk of rejection, that’s actually false. The medical research of the last few decades where cord blood has been used to treat pediatric and other cancers shows that you do need to match cord blood to the patient (4,5). What happens if you don’t match? There’s a risk of a serious disease called GVHD, which can start with a skin rash and end in organ failure. Meaning a clinic that isn’t HLA type matching umbilical cord blood units to the patient receiving them through complex and expensive blood tests is placing patients at risk.
Next, let’s take the second statement that the stem cells are “neutral” which means they have no DNA. Well, we know there are no live and functional stem cells being delivered at Stem Cell Centers, but the webpage describes a live stem cell therapy, so let’s play along. First, all living cells have DNA. That’s how your body decides what the cells should be and how the machinery should function (8). In fact, cells without DNA wouldn’t be alive, they would be dead. Finally, DNA is not directly involved with identifying whether a cell is foreign or if that cell would cause rejection, as that’s actually the bar code system I discuss above. Hence, this statement is demonstrably false. In fact, it’s so bad, it seems like it was written by someone without any medical or scientific training.
What Are These Clinics?
I love to do what I called the “reverse lookup” on these clinics. Hence, I got the address for the local clinic listed on the Stem Cell Centers web-site and highlighted it and right-clicked and chose “Search Google”. What came up? A chiropractic clinic. While you may want to see your chiropractor for back pain and manipulation, it’s not likely the best place to get an injection of stem cells. Why? There is little expertise in performing the complex injections needed to perform stem cell therapy in the right way. In addition, my review of the research showing that whichever products Stem Cell Centers is actually using (umbilical cord blood or amniotic fluid), neither product has live cells, is emblematic of why your chiropractor’s office is not the place to get this done.
The upshot? So back to the Stem Cell Centers review. As you can see, it wasn’t hard to dive deeper on Stem Cell Centers to see that what they promise and what they’re delivering are two different things. In addition, there isn’t even a basic understanding of biology shown on their website. Finally, when looking at my local clinic, this is not a medical clinic with experts who might know how to safely and precisely inject stem cells to help your problem, but a chiropractic clinic in a strip mall.
_______________________________________________________________________
References:
(1) Dustin R. Berger, Nicolette F. Lyons, and Neven J. Steinmetz. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Liliya Becktell, Andrea Matuska, PhD, Stephanie Hon, DVM, Michelle L. Delco, DVM, PhD, Brian J. Cole, MD, Lisa A. Fortier, DVM, PhD. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A. J., Hirahara, A. M., Andersen, W. J., Rothenberg, J., & Fierro, F. (2019). Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. https://www.ncbi.nlm.nih.gov/pubmed/15191952
(5) Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood Jul 2014, 124 (3) 363-373; DOI: 10.1182/blood-2014-01-514786
(6) Lee SJ. Classification systems for chronic graft-versus-host disease. Blood Jan 2017, 129 (1) 30-37; DOI: 10.1182/blood-2016-07-686642
(7) Mosaad, Y. M. Clinical Role of Human Leukocyte Antigen in Health and Disease. Scand J Immunol, 2015, 82: 283-306. doi:10.1111/sji.12329
(8) Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. The Structure and Function of DNA.Available from: https://www.ncbi.nlm.nih.gov/books/NBK26821/

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.

