StemShot New Product Information Review
Last week we learned that “StemShot” was actually a trademark and not a specific product. Looks like the medical distributor that holds the trademark pulled its use by the Utah Cord Blood Bank and gave it to a new cord blood supplier. A colleague sent me the new marketing materials this week, and they continue to not accurately represent what’s in the bottle. Let me explain.
What Is Cord Blood?
Cord blood is harvested from the umbilical cords of live births. It’s been theorized that cord blood may be helpful in regenerative medicine, but few clinical trials exist to prove that point, especially in orthopedics. In addition, most of the research done to date is actually on cord blood that has been processed and had stem cells isolated and culture expanded for a few weeks. The problem? This is illegal in the United States, so what we get is cord blood taken from umbilical cords and thrown in bottles.
Does It Contain MSCs?
Mesenchymal stem cells (MSCs) are kind of the all-around decathlete of stem cells. Like a super athlete, they’re good at many different types of tissue repair, including those we focus on in orthopedics. Hence, it’s not surprising that cord blood vendors try to convince doctors that their products contain loads of MSCs. However, there’s one little problem. Reality. You see, the research on cord blood shows that it only contains rare MSCs, and many cords contain no detectable MSC levels that result in any ability to culture cells. See my video below for more information on this topic:
What Do Cell Viability Numbers Mean?
One of the things that really confuses physicians is the concept of cell viability. They really don’t get that the test that amniotic and umbilical cord tissue vendors provide is a simple live/dead stain that says little about the overall health and function of the cells. To understand more about why this is, see my video below:
What Is Flow Cytometry in the Context of Testing Umbilical Cord Products?
If simple live/dead viability stains confuse doctors, flow cytometry data flies right over their heads. Why? No musculoskeletal residency or fellowship program teaches how to interpret these tests. Here I’ll look at what StemShot is sending doctors and examine whether they can support their contention that this data supports that their product has MSCs. However, first, let’s learn the basics of how to interpret flow cytometry.
Flow cytometry is the use of LASERs to ID markers on cells that have been stained with a fluorescent antibody. Different types of cells have different cell-surface markers, so the patterns of these can identify a cell. To identify an MSC, you need a whole bunch of markers on a cell that should be there and a whole bunch more that shouldn’t be there. To learn more, see my video below:
The Markers Tested by StemShot Are Inadequate to ID MSCs
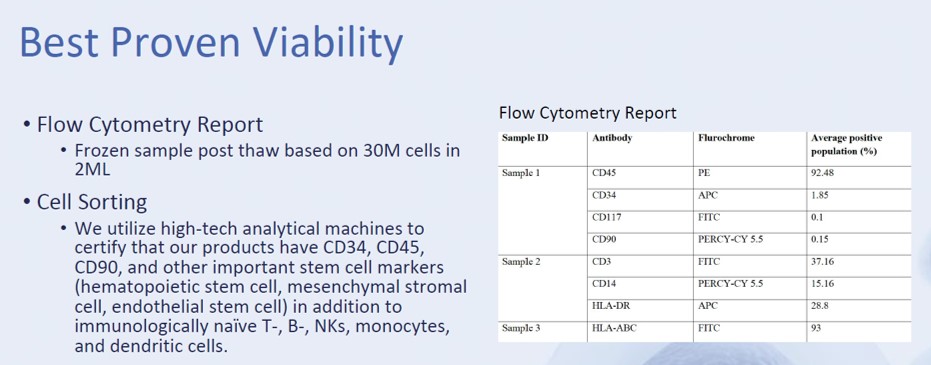
The flow cytometry testing that StemShot is sending around to doctors states the following:
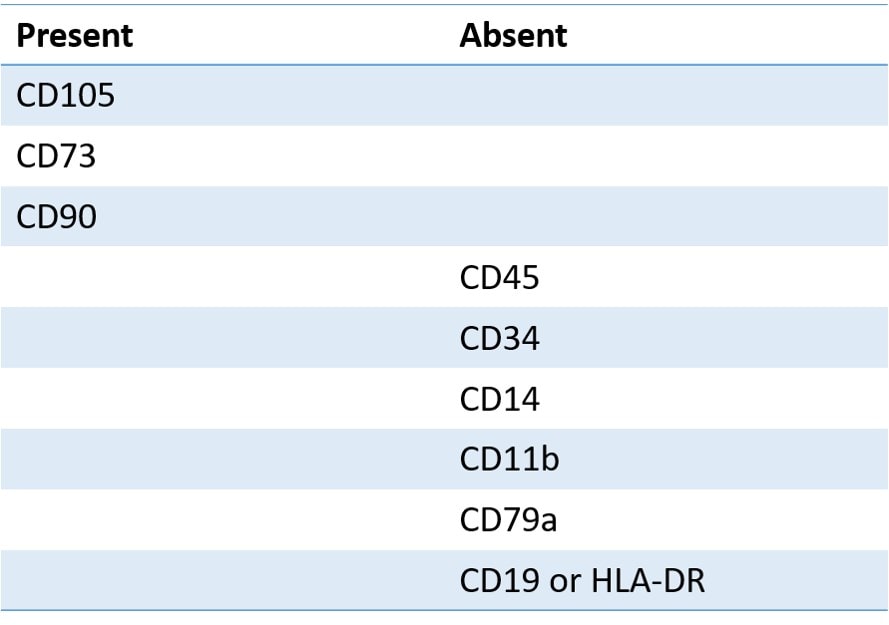
So what does this mean? When it comes to identifying stem cells, not much. In order to ID a single MSC, you would need the following:
So here we see that we need to test for the marker known as CD105. Did the StemShot report test for this marker? Nope. You also need to test for CD73; was it tested? Nope. In addition, many of the markers they state are positive in a large percentage of the sample shouldn’t be present AT ALL.
Also note that the product distributor is stating that they “use high-tech analytical machines to certify that our products have CD34, CD45, and CD90, and other important stem cell markers (hematopoietic stem cell, mesenchymal stromal cell…” So are these really “stem cell markers”? Not so much. CD34 is a marker that identifies hematopoietic stem cells. However, CD45 is a common white blood cell marker and needs to be NEGATIVE (i.e., not present) on MSCs. Many cells express CD 90, including thymocytes (precursor of T cells in the thymus) and CD34(+) prothymocytes, neurons, mesenchymal stem cells, hematopoietic stem cells, NK cells, murine T-cells, endothelial cells, renal cells, follicular dendritic cells (FDC), and a fraction of fibroblasts and myofibroblasts. So two of these markers ID an MSC like the color red in a car identifies a Ferrari. Meaning there are many red cars that aren’t Ferraris, and there are many cells that have these markers outside of stem cells.
Which Critical Lab Tests Did StemShot Leave Out?
Flow cytometry data is always interesting, but there are two more scientific standard tests that need to be done that StemShot left out. These are defined by the Foundation for the Accreditation of Cellular Therapy (FACT) guidelines. In these tests, the cells need to be cultured and form plastic adherent colonies and then need to be differentiated into different cell types in culture. Were these tests done? Nope. For more info, see my video below:
The upshot? As discussed, StemShot isn’t so much a product where the manufacturer did extensive lab and clinical research into the best practices for processing cord blood and how that impacted patients; it’s more of a marketing trademark held by a distributor who contracts with a cord tissue supplier. The most recent data being sent to doctors stretches the truth and leaves out critical tests needed to identify MSCs. Which is par for the course of all of these amniotic and umbilical cord tissue vendors, so these issues are not specific to StemShot but emblematic of industry-wide failures.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.