Using Machine Learning to Predict Stem Cell Knee Arthritis Outcomes
One of the most frustrating things about stem cells and regenerative medicine is the difficulty in predicting which patients will respond with “home run” results and which won’t respond at all. You would think from looking at the miracle-cure stem cell websites popping up around the Internet that stem cells are magic pixie dust able to cure whatever ails a human. However, that’s not the case at all, in that all stem-cell-based treatments have a success and failure rate. To fix that vexing problem, we’ve been working for years on predictive models that help Regenexx providers decide who is more likely to respond. Our newest model now takes this to the next level by measuring the levels of 25 cytokines and growth factors from stem cell knee patients and inputting this into a predictive model based on machine learning.
What Is Machine Learning?
The human brain is great at deciphering patterns. Take, for example, that your brain can easily recognize thousands of faces. This would seem like a trivial task, but until recently, despite that fact that a computer can process information millions of times faster than your single neurons in your brain, it was awful at recognizing faces. This is because your brain and today’s computers are built quite differently. A computer processes visual information in serial (one piece at a time), and your brain processes the whole picture all at once.
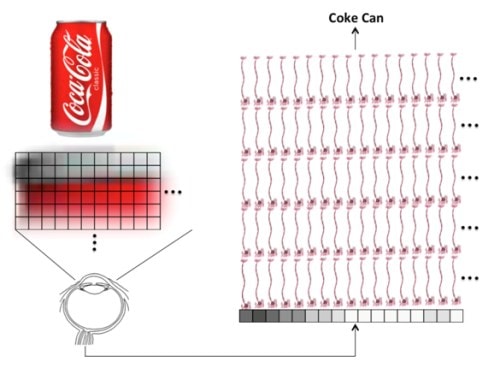
A computer using traditional software is awful at recognizing Coke cans. It needs to inspect each and every pixel in the image and compare that to some known picture of the object. Processing that information can take more time than it takes your brain. In addition, throw the computer a variation by letting a shadow fall across the can and it now thinks the can is something else.
Back in the ’60s, in the golden age of early computing when the machines occupied whole rooms, computer scientists believed they were on the verge of creating a human brain in a box. Their hopes were quickly dashed as they realized that tasks like recognizing a Coke can with the technology available back then could take a computer several days. Some smart scientists reasoned that if we designed software with layers of artificial neurons, we could sort of model how the brain processed information. This software approach was called a neural network or machine learning. However, this field labored in obscurity until a seed change happened about five years ago. At this point the big money in companies like Google, Microsoft, Facebook, and others began to realize that they needed machine learning if their products were to ever see their full potential. For example, you may have seen the fanfare behind Google announcing that its image software had been taught to recognize a cat! While this seems trivial, for a software program based in machine learning, it was a big deal.
Predicting Stem Cell Knee Arthritis Outcomes
We’ve tracked the outcomes of every treated patient in a registry for many years. In fact, we’re the only clinic using stem cells that has done this to date. One of the things that we’ve been able to use all of that data for is to help Regenexx providers determine who is a good or poor candidate for stem cell therapy. However, some things that we thought would be associated with outcome, like the severity of arthritis on an MRI, turned out for some joints not to be related. I’ll never forget spending two weeks pulling out all of the minute details of knee MRI images on patients for whom we knew the final outcome of the stem cell therapy, only to be horribly disappointed that none of it correlated with that outcome!
After the knee MRI fiasco, I knew we needed more data to plug into our predictive model. One commonsense way to do this occurred to me while in our garden. Every industrial farmer on earth knows that the soil conditions must be right if your seeds are to reach their full potential. Could we do the same thing for knees by measuring the status of the normal fluid in the joint, which in effect is the same as the soil in which our stem cell seeds were to be planted? Thus began our knee microenvironment study, measuring 25 different cytokines and growth factors in the knee before and after every Colorado stem cell knee procedure for knee arthritis.
A Glimpse at the Future
Collecting 50 data points on every patient of obscure chemicals inside the knee quickly produces a data set where patterns can be really hard to recognize. Should TGF-beta go up or down? How about anticatabolic molecules like TIMP, A2M, or IRAP? When we first got the data back from the pilot phase of this IRB-approved study, it was difficult to see any specific patterns. Given that no one has ever attempted to use a large panel of the levels of these chemicals in the knee to predict the outcome of treatment, there was no guidebook. That’s when it hit me that we needed a neural network, which can detect patterns in chaotic data that a mere human might miss.
This week, we got back our first result on a predictive model using only the first 14 patients for whom we had an outcome. Our biostats expert trained the system about 100 times on randomly chosen patients with various marker levels, and we ended up with a system that was 67% accurate in predicting outcome. Given that we now have 43 patients in the pipeline awaiting a final outcome measure, by the early summer we should have a system that gets better and better at predicting outcome based on the quality of the soil and know which of these growth factors is the most responsible for that outcome. By the end of the year, we could have an interesting tool that may help Regenexx providers more accurately determine who is a good and a poor candidate for a stem cell knee procedure.
The upshot? Our knee microenvironment predictive model is a great example of the personalized healthcare revolution coming our way. From a pragmatic research standpoint, it should help Regenexx providers determine who they should dissuade from a stem cell knee procedure. From a scientific standpoint, it’s exciting to see how software designed to recognize cats on the Internet may help patients get better care!

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
