The New VAST Trial Results on VIA Disc
It’s my job as CMO of Regenexx to stay on top of the latest and greatest technologies out there that can be used to treat Degenerative Disc Disease in the spine. Today I’ll tackle one called VIA Disc. My goal is to dissect a recent and past paper to see if this is something we want to add to our Regenexx network offerings. Let’s dig in.

It’s My Job
I love that Jimmy Buffet song, “It’s My Job”:
My job as Chief Medical Officer for Regenexx is to make sure we stay well ahead of the curve in Interventional Orthobiologics. Meaning as new stuff comes along I have to perform a deep dive to see if it’s better than what we’re using. I’ve done that many times in the past on various technologies.
The product for today’s analysis is called VIA Disc. Frankly, while I knew it was out there until a colleague send their most recent paper, I couldn’t have told you much about it.
What Is VIA Disc?
VIA Disc is made from ground-up donor disc tissue plus saline (1). Or at least that’s what’s on the Vivex Biologics website. Meaning from that description, it’s somebody else’s disc tissue injected into your degenerated disc. However, I have found other descriptions online including this one (10):
“VIA Disc Matrix (Vivex Biomedical) is composed of human disc tissue donated from cadavers with viable cells. It consists of a nucleus pulposus allograft suspension that is mixed with a minimum of 6 X106 cryopreserved cells. The cell source and method of processing has not been disclosed.”
I would say that the “has not been disclosed” part is accurate. Meaning I found lots of language about donor disc material and viable cells, but no reports of exactly what’s being delivered, even in scientific papers. I also found no data on the viability of this material (i.e. a live/dead or CFU-f count).
The manufacturer claims to be able to supplement your disc tissue with material that can cause it to again hold onto water:
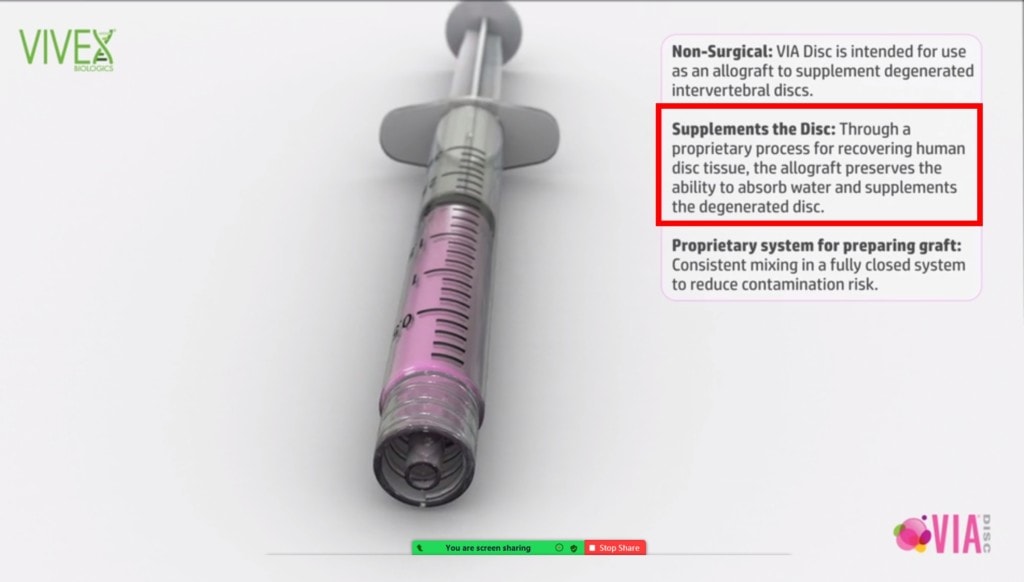
That ability in the healthy disc is mediated by GAGs (glycosaminoglycans) and VIA Disc should have GAGs because one ingredient appears to be the ground up Nucleus Pulposis of donor discs.
The procedure surrounding VIA Disc involves injecting this donor disc material into the degenerated disc via a fluoroscopically guided injection with a 22 gauge spinal needle. Given that a similar technique is used by Interventional Pain Management (IPM) physicians to perform Discography, this can be done by just about any qualified IPM trained doctor.
The Data
A colleague asked me to check out VIA Disc based on a new study they had published which was the 12-month result of their VAST trial (2). That multi-center trial was published in September of 2021 and included 218 patients with back pain caused by 1 or 2 level Degenerative Disc Disease, 36 of which were lost to follow-up. The authors compared VIA Disc injections to saline and there was a conservative care group that was allowed to cross over to the treatment at 3 months.
So how did VIA Disc do versus saline? Not that great honestly. Let’s dig into the data.
Below is a graph I created from the paper’s data. The solid lines represent the VIA Disc and saline control groups. Blue is the treatment and red is the control:
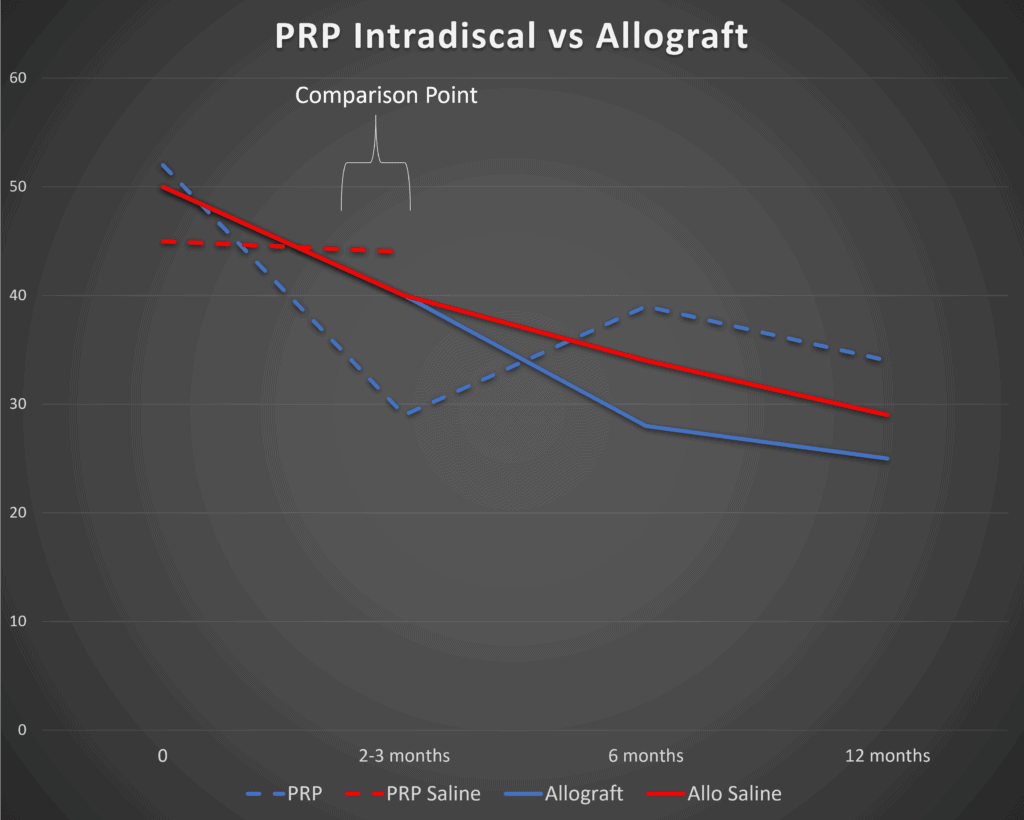
So right off the bat, we see some serious problems. Note at the 3 months mark (marked 2-3 months here) that there is zero difference between the VIA Disc and saline injections (solid lines). A small difference between the groups (which we will discuss later) doesn’t show up until 6 months.
Since we already have a well-done RCT on the use of Platelet-Rich Plasma (PRP) injected intradiscal, it’s certainly fair game to compare the two, which is what I did in the above graph (3). To create this comparison I took the Oswestry Disability Index results from the VAST trial and graphed those next to the Functional Rating Index results from the PRP trial. Given that both are 0-100% scores, they can live on the same diagram. However, because they’re different functional questionnaires measuring disability from 100% (totally disabled) to 0% (no disability due to back pain), it’s more proper to compare the difference between the saline and control groups within each study and not the results between the studies.
Note that right off the bat, when we compare the PRP data (dashed lines), it looks quite a bit better. Meaning that PRP clearly separates out from saline injections at 2 months with about a 14% difference in FRI scores (blue is PRP and red is control). Again, at 3 months, the VIA Disc has shown no difference at all from the saline control.
So what can we conclude? PRP seems to work much faster than VIA Disc, or at least in these two studies.
MCID Problems
What is an MCID? That’s the minimal clinically important difference. This concept exists because while two groups in a study may be statistically different, the differences may not be enough for patients to notice. Hence the MCID is that change in an outcome metric when patients begin to say that the results are meaningful to them.
At the end of 1-year, the difference in the Oswestry questionnaire between VIA Disc and saline control is about 4 points based on my reading of their graphs (they don’t seem to show that data in a table). However, the MCID for the Oswestry is about 9 to 14 points based on the published data (4-6):
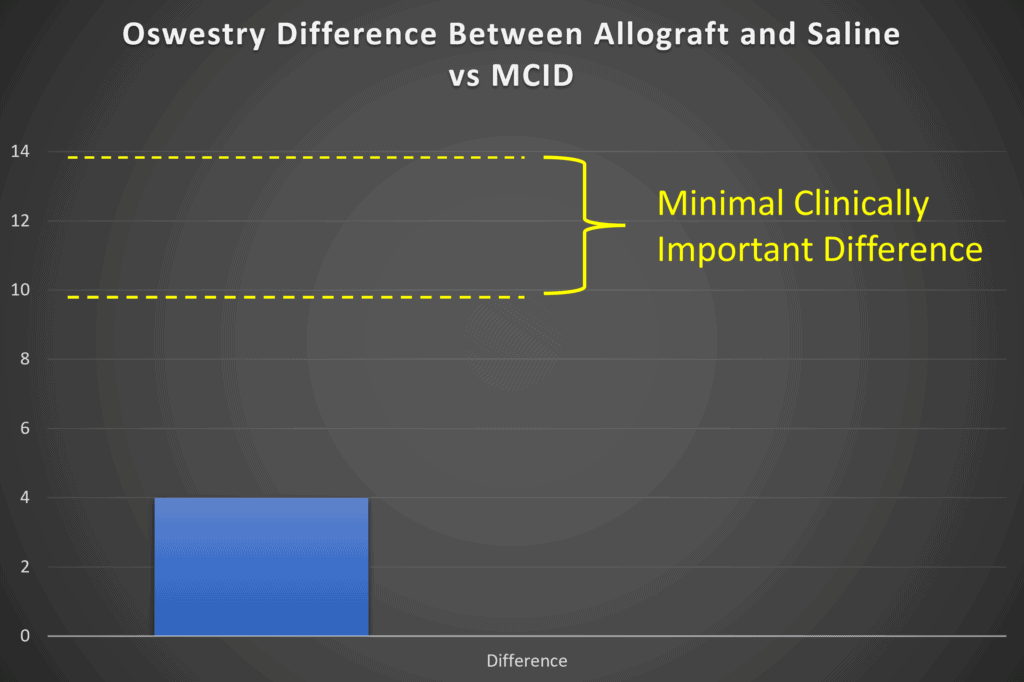
Hence the mean difference between the groups is not clinically meaningful. Meaning while it may be statistically different, the average patient couldn’t tell the difference between getting their disc injected with VIA Disc or saline when it comes to functional improvement.
Subgroup Analysis or “Plan B”
Every researcher that conducts a clinical trial hopes that the treatment group will be different enough from the placebo that you can beat the MCID when you compare the means. I know, because I’ve performed 3 RCTs that have been published, have a fourth underway, and are about to start a 5th and 6th. However, when that doesn’t happen, as was the case in the VAST trial, you have to go to plan B. What’s that? It’s called a responder or subgroup analysis.
While the mean or average results may not be all that different between the groups, maybe if you take the percentage of responders in the treatment group, that will be greater than the percentage in the placebo group. That’s exactly what the authors did in this paper (I recreated one graph from the paper below as the quality of the PDF blow-up was poor):
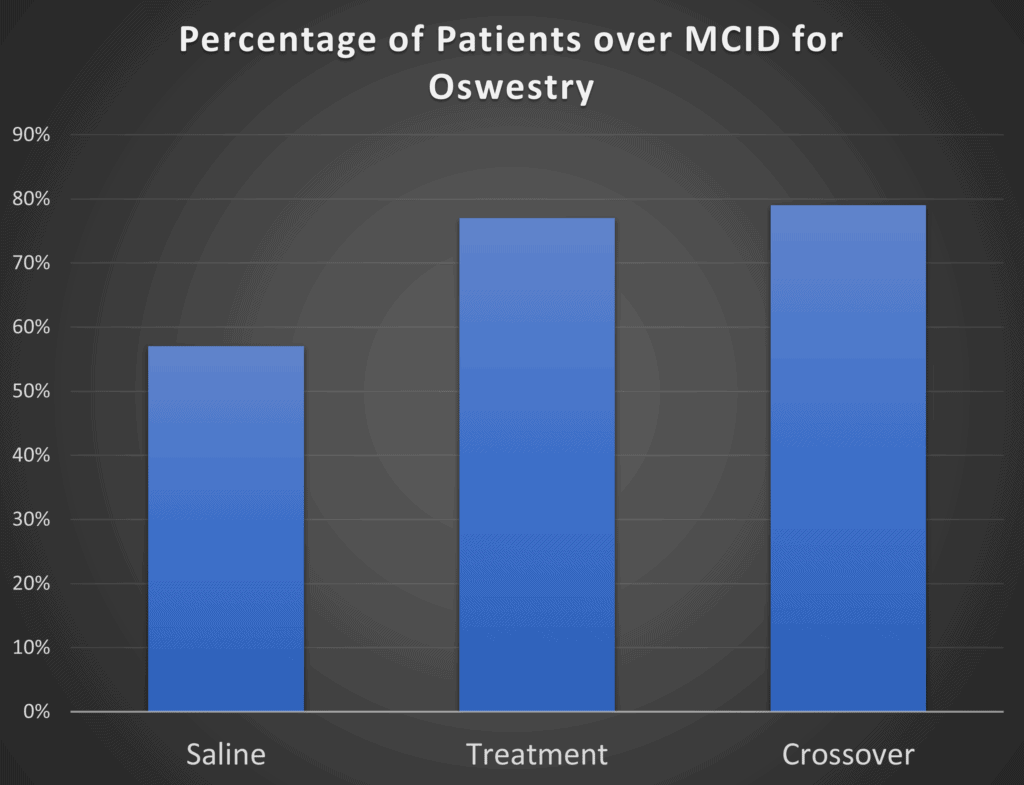
Here the authors took the percentage of patients that showed an Oswestry score difference >15 points. The problem? Almost 6 in 10 patients who got a saline placebo reported that level of functional improvement. So the VIA Disc treatment only had about 20% more responders than the control group. The good news? This looks better than the group mean differences above. The bad news, it’s doesn’t look all that much better.
Conclusions on the VAST Trail
If VIA Disc works, it seems like it’s probably less effective than PRP in the short run. Certainly, PRP packs a bigger punch against placebo in the first few months. My concern is the fact that at 6 months and 12 months is not that much more compelling than just injecting the disc with saline.
Past Studies – MRI Improvement?
The authors above had published a prior study where they looked at the first 24 patients in the above trial for which the pre and post-imaging (x-rays/MRIs) were available (7). After analyzing that data, this is the curious statement made in that abstract:
“Measurement of disc height demonstrated a net increase for all subjects randomized to the active allograft; total disc heights of the allograft treated increased from 40.15 to 41.58 compared to those treated by placebo 40.01 to 41.08.”
So the authors are saying that a VIA Disc injection increased disc height more than a saline injection. That could be important because it may indicate that the VIA Disc material is helping the disc hold onto water better. However, let’s take a closer look at that claim.
I graphed that data as this appears to be a pixel measurement. Below you can see how ridiculous the claim looks:
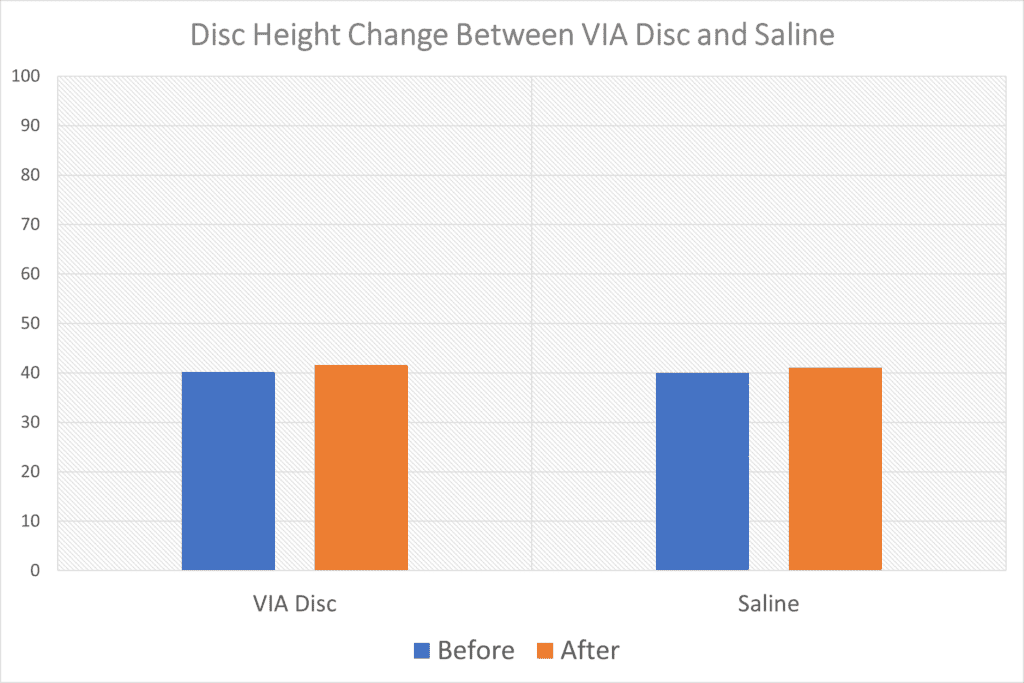
Can you tell the differences between the change in height of the VIA Disc treated discs on the left and the saline-treated ones on the right? Meaning whatever differences they found were likely well within the accuracy of the measurement technique used. In addition, since this is only an abstract, we also don’t know critical things like when these images were taken (they would have to be taken at the exact same time of day as the discs gain height as you sleep and lose it as you sit).
This statement in the abstract probably shows us that whatever they measured was an artifact:
“Retention or increase in disc height was related to the number of levels treated; those subjects treated at 2 levels increased disc height not only at the levels treated but at all intervertebral disc levels rostral to the intervention.”
Meaning that in some patients, untreated discs also increased in height! Given that there is no mechanism for that to happen, again, this is likely not a treatment-related finding.
Finally, these measurements didn’t appear in the full VAST Trial publication, so you can bet that if this were a promising finding of being treated with VIA Disc, the authors would have continued to take MRIs and sung this result from the rooftops in their 2021 paper. Hence, as an expert, I’ll conclude that VIA Disc likely doesn’t improve the health of the disc in a way that can be easily seen on MRI.
Medicare Coverage?
In researching Via Disc reimbursement, the company’s 2021 coverage guide suggests that there is Medicare coverage under CPT code 0627T (8). However, the IAASS website states (9):
“Because the new codes are Category III, no relative value units are set for the procedures, and providers are expected to provide estimated costs to payers for reimbursement. ISASS encourages physicians performing the service to determine their practice costs for the service and bill accordingly with the Category III code(s).”
What are class III codes?
“CPT Category III codes are a set of temporary (T) codes assigned to emerging technologies, services, and procedures. These codes are intended to be used for data collection to substantiate more widespread usage or to provide documentation for the Food and Drug Administration (FDA) approval process.”
What does all of that mean? Basically that VIA Disc has the same type of T code that PRP has which is not reimbursed by Medicare. I searched the major Medicare contractors including Noridian, First Coast, and Novitas, and was unable to find anything about the reimbursement of code 0627T. I also found many private carriers that consider Via Disc experimental and don’t offer coverage.
Regulatory
I found this entry in a Blue Cross Blue Shield coverage determination for Louisianna (10):
“…it is not clear if VIA Disc Matrix meets the U.S. FDA criteria for what is considered minimal manipulation and homologous use for human cells, tissues, and cellular and tissue -based products (HCT/Ps).”
That’s a much different description than what’s on the VIA Disc website. The product is currently labeled as a 361 tissue, but given the description above of Vivex distributing live cells and not disclosing the processing, the regulatory status of this product, IMHO, is an open question.
Cost?
I wasn’t able to find much about the cost of the product. However, I reached out to some colleagues who have enquired. The cost of the product is approximately $6-12,000 USD. That’s MUCH pricier than PRP. In addition, given the lackluster showing when compared head to head against PRP and saline, I personally can’t justify that kind of price point.
Intangibles
Despite not being convinced by the results from the pivotal trial done by Vivex on VIA Disc, I want to congratulate the company for performing an RCT. All too often in the Stem Cell Wild West, that never happens. So well done Vivex for making this investment!
The upshot? After this deep dive into VIA Disc, at this point, without more convincing data, I won’t advise that Regenexx add it to our network’s corporate-covered offerings. Perhaps newer long-term data will be more convincing that VIA Disc can outperform cheaper Orthobiologics. Or more data will show that VIA Disc is making low-back discs better over time? I await more research.
_____________________________________________
References:
(1) Vivex Biologics. VIA Disc NP Allograft. https://gotviadisc.com/about-via-disc/ Accessed 2/21/22
(2) Beall DP, Davis T, DePalma MJ, Amirdelfan K, Yoon ES, Wilson GL, Bishop R, Tally WC, Gershon SL, Lorio MP, Meisel HJ, Langhorst M, Ganey T, Hunter CW. Viable Disc Tissue Allograft Supplementation; One- and Two-level Treatment of Degenerated Intervertebral Discs in Patients with Chronic Discogenic Low Back Pain: One Year Results of the VAST Randomized Controlled Trial. Pain Physician. 2021 Sep;24(6):465-477. PMID: 34554689.
(3) Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, Harrison JR, Gribbin CK, LaSalle EE, Nguyen JT, Solomon JL, Lutz GE. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM R. 2016 Jan;8(1):1-10; quiz 10. doi: 10.1016/j.pmrj.2015.08.010. Epub 2015 Aug 24. PMID: 26314234.
(4) Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008 Nov-Dec;8(6):968-74. doi: 10.1016/j.spinee.2007.11.006. Epub 2008 Jan 16. PMID: 18201937.
(5) Johnsen LG, Hellum C, Nygaard OP, Storheim K, Brox JI, Rossvoll I, Leivseth G, Grotle M. Comparison of the SF6D, the EQ5D, and the oswestry disability index in patients with chronic low back pain and degenerative disc disease. BMC Musculoskelet Disord. 2013 Apr 26;14:148. doi: 10.1186/1471-2474-14-148. PMID: 23622053; PMCID: PMC3648434.
(6) Monticone M, Baiardi P, Vanti C, Ferrari S, Pillastrini P, Mugnai R, Foti C. Responsiveness of the Oswestry Disability Index and the Roland Morris Disability Questionnaire in Italian subjects with sub-acute and chronic low back pain. Eur Spine J. 2012 Jan;21(1):122-9. doi: 10.1007/s00586-011-1959-3. Epub 2011 Aug 8. PMID: 21823035; PMCID: PMC3252446.
(7) Ceall D. The Spine Journal. 214. Viable allograft supplement for disc degeneration: measurable disc height increases at 12 months in first 24 subjects. VOLUME 20, ISSUE 9, SUPPLEMENT , S106, SEPTEMBER 01, 2020. DOI:https://doi.org/10.1016/j.spinee.2020.05.625
(8) Vivex Biologics. CODING AND BILLING GUIDE JUNE 2021 REIMBURSEMENT INFORMATION. https://gotviadisc.com/wp-content/uploads/2021/06/VIV1282_VIADISCNP_MKG-IVP-9_R1_060821.pdf Accessed 2/21/22
(9) ISASS. New 2021 Category III CPT Codes for Allogenic Disc Matrix Material. https://isass.org/new-2021-category-iii-cpt-codes-for-allogenic-disc-matrix-material/ Accessed 2/21/22
(10) BCBS of Louisiana. Allograft Injection for Degenerative Disc Disease-Policy # 00750
Original Effective Date: 11/01/2021. https://providers.bcbsla.com/-/media/Files/Providers/00750%2020211101%20Allograft%20Injection%20for%20Degenerative%20Disc%20Disease%20pdf.pdf Accessed 2/21/22

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
