Why Counting Platelets in High-Dose PRP Can Be a Fool’s Errand
There’s a huge current trend in more advanced clinics to buy a hemoanalyzer and count the platelet content of the PRP produced by the clinic. While on the surface, this would seem to be a noble effort, the devil is always in the details. Regrettably, the fine print on this one shows that most of these counts are likely more fiction than fact. Let’s dive into the science to see what’s going on.
PRP and Platelet Counts
When I founded Regenexx in 2005, we had some basic science observations from our lab that were hard to ignore. Basically, when using the patient’s platelet lysate to grow their mesenchymal stem cells in culture, in middle-aged and older patients, the higher the platelet dose, the better the growth of their stem cells. Hence, when we began using platelet-rich plasma (PRP), we knew that for most of our patients, a higher dose was better. At the time, however, the few clinics that were offering PRP were using off-the-shelf systems that could only produce low-dose PRP. Fast forward almost two decades and the current wisdom based on in-vitro studies and clinical trial results confirms that higher-dose PRP beats lower-dose PRP (2-6). As a result, we see many more advanced practices beginning to count the platelet content of their PRP.
At first blush, buying a machine to count the platelet content of your PRP seems to be a best practice sort of thing, right? It shows an additional devotion to the art that can’t be denied. Having said that, like everything else in life, the fine print or details often get in the way of the best of intentions.
Platelet Counting 101
First, a machine that counts blood components is called a hemoanalyzer. These machines are designed specifically to count platelets in whole blood, and that little factoid will become important as we explore how they work.
A commonly used hemoanalyzer machine today is one produced by Horiba. The Micros range of products is often sold to clinics to count platelets in PRP. They use an impedance method to identify and count cells. This means that cells in an electrolyte solution are passed one by one through a small aperture surrounded by two electrodes. As they pass through, changes in voltage are recorded, indicating cell count and size.
However, impedance counters (and all automated cell counting methods for that matter) have a problem. This is from a guide to cell counting technology (1):
“Because these instruments count cells one-by-one, aggregated and complex cell types cannot be counted by these instruments.”
This problem is counting aggregates, which will become very important as we look at counting the platelet content in PRP.
Platelet Aggregates 101
Platelets are built to aggregate and cause a blood clot. While adding anticoagulants like ACD will help reduce those aggregates, the more platelets are concentrated and agitated by the process of making high-dose PRP, the more likely they are to aggregate anyway. The biggest issue for accurate platelet counts is when two or three platelets stick together (doublets or triplets). These small aggregates will now have a size and impedance more similar to a red or white blood cell than a platelet, so they get miscounted as something else. In this case, the platelet count reported by the machine goes down, while the red or white blood cell count goes up.
A Confused Doctor
One of our affiliates recently bought a Horiba hemoanalyzer because it’s the same one we use in the CSC and Regenexx labs. He was confused because his counts seemed off, and he knew that some of this was happening due to aggregates. This was the answer of Dustin Berger, one of our scientists, which contains a critical message for all physicians involved in platelet counting:
“What you describe is not uncommon. The more concentrated a PRP becomes, the greater the prevalence of platelet clumping. These clumps are subsequently read as lymphocytes by the Micros60, resulting in lower platelet counts and higher WBC counts than what is truly present.
The following plots show platelet counts from 4X, 10X, and 20X PRPs (mean +/- SD, n=10). Note at the 4X concentration that there’s ~4-fold increase in platelet concentration…however, at the 20X concentration, there’s only a ~15-fold increase. As you’ve stated, this is not due to lost platelets, but platelet clumping.
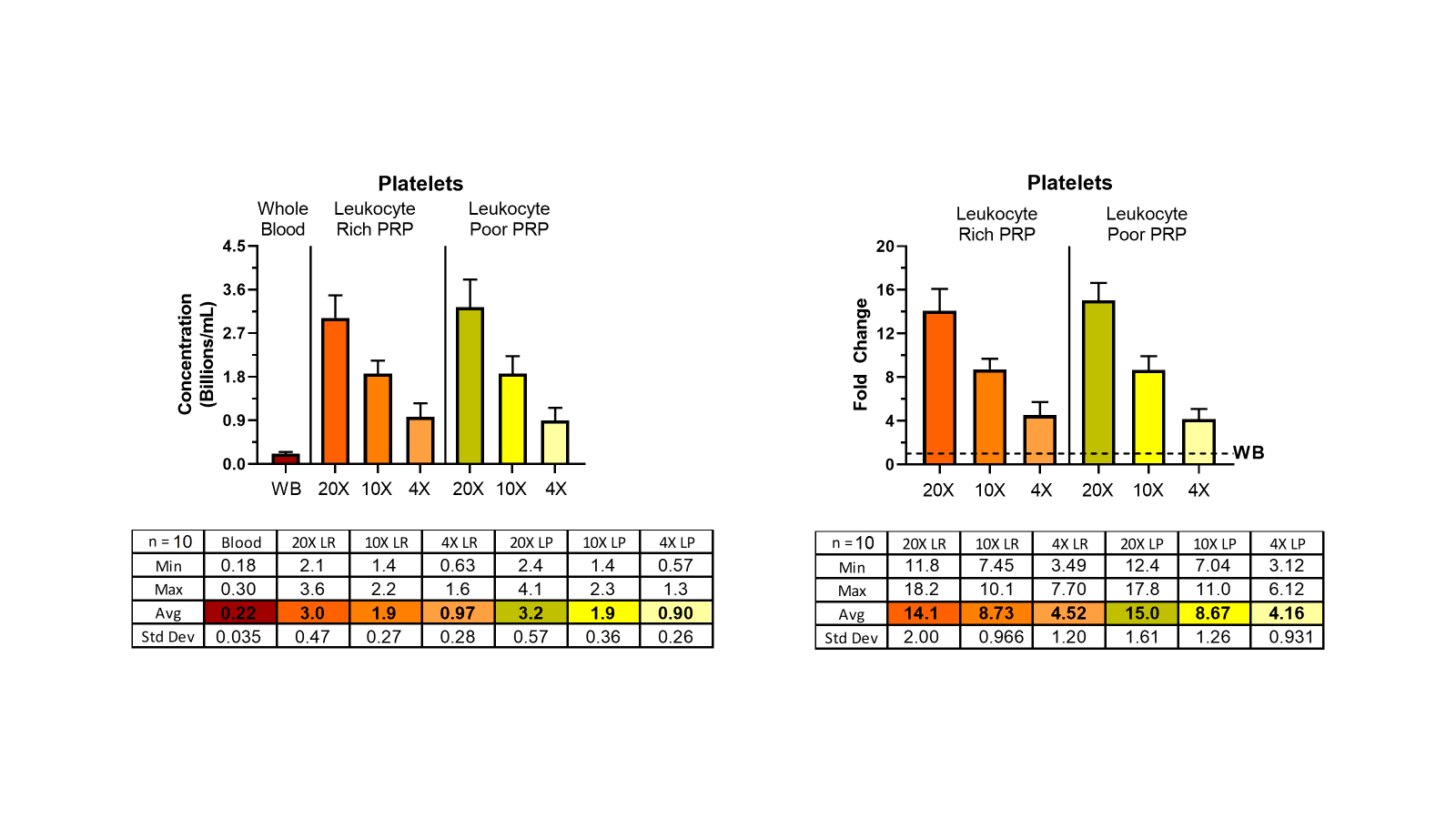
Moreover, we’ve been able to confirm clumping by adding the platelet aggregation antagonist PGI2 (prostacyclin) at 1uM and observing an increase in platelet counts with a concomitant decrease in WBC counts. However, this is not a clinical solution.
Your approach to counting seems sound. I don’t have a lot of personal experience vortexing cellular samples…I was always cautioned against it in graduate school. Though there is clearly published precedence for doing so to reduce platelet clumping prior to counting.
https://academic.oup.com/labmed/article/28/10/665/2503680
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10064346/
One thing you could do would be to count plasma prior to concentrating and PPP post concentrating to get a better picture of total number of platelets in the PRP, but that doesnt address the clumping issue.”
The Bottom Line on PRP Counting
There is no practical way to get an accurate platelet count of high-dose PRP using a hemoanalyzer. While you can help the count by performing tricks like vortexing or counting lost platelets in the platelet-poor plasma, you will always be at the mercy of a machine never designed to count PRP.
So, while I’m not against advanced practices buying hemoanalyzers and counting platelets, we all need to realize that the data produced by these efforts is flawed.
Could Hemoanalyzers be Useful?
If the goal of these units is to define a minimum platelet count to create a floor for treatment quality, then they could help with that effort. For example, if we assume that the machine will underestimate the platelet count of high-dose PRP, but we place a floor on that count of X billion, then might be a good idea. For example, several papers have suggested minimum platelet counts are associated with better outcomes. However, it should also be noted that the platelet counts in these studies, if a hemoanalyzer was used to measure these counts, are likely inaccurate as well. Hence, we really don’t understand with certainty where that floor lives.
Maybe Flow Cytometry Can Help?
Our lab has owned three flow cytometers over the years. We currently use a rare event flow cytometer, and I have worked closely with our scientists for years to interpret the data these machines produce. On LinkedIn, in the discussion about this post, several people have stated that using flow cytometry could somehow resolve this issue of counting platelet aggregates. Let’s look at that problem.
Flow cytometry is not for the faint of heart. This is not a machine like a hemocytometer, which produces neat little reports showing the number of x cells or y cells. Instead, you stain the cells with a fluorescent antibody, which attaches to certain cell surface markers. Those cells are then forced through a small aperture, and the number of cells that light up for that antibody stain are counted, and the brightness of that signal is noted. All of that data gets placed on a scatterplot that looks like this:
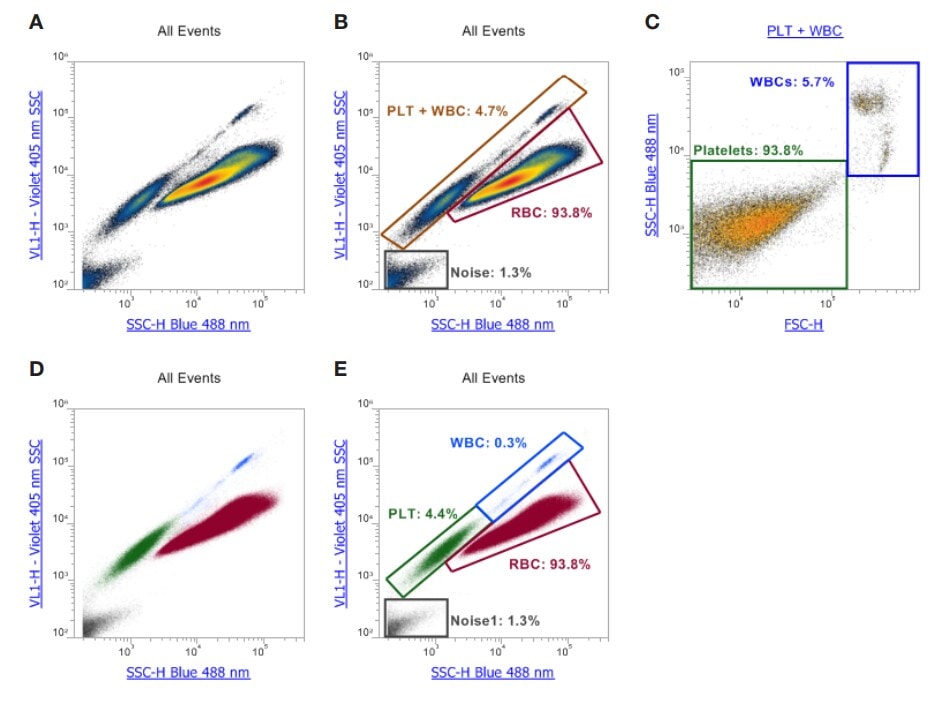
These are platelet scatterplots for the machine we own. Without getting too much into the weeds of various channels and side and forward scatter, each dot plotted is a “hit” or platelet/cell recorded as it went through the small opening. The large smudges you see are made up of many, many hits.
Now, it has been proposed by some on Linkedin that we could somehow use this type of data to accurately ID platelet aggregates and get to an accurate platelet count despite that aggregation. Let’s simplify this to see why that won’t work.
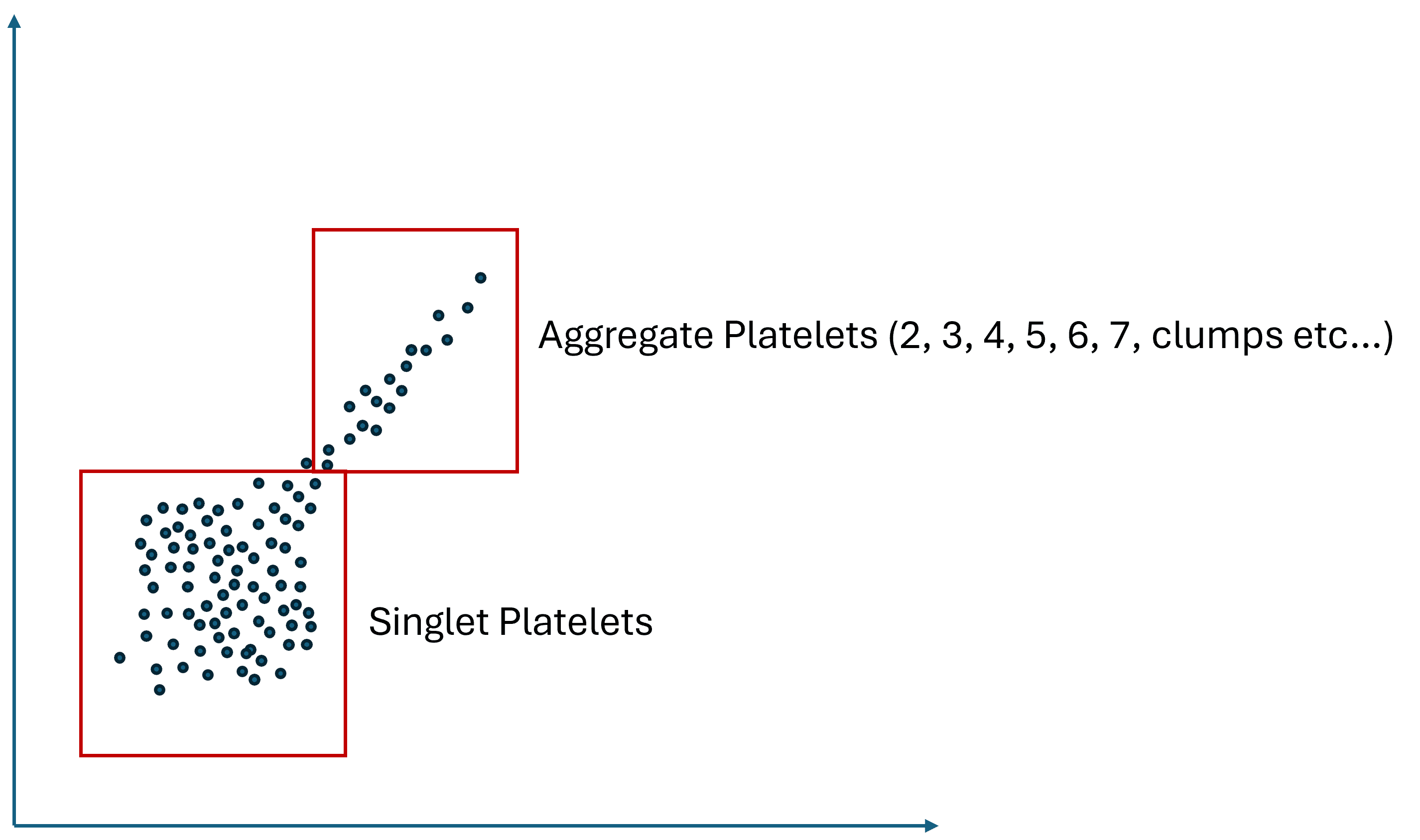
Again, without diving too deep into an immensely complex subject, I know enough about flow results to understand that you will get a scatterplot like the image I created above. That means you will have a singlet population you can count for platelets and a smudge of platelet aggregates that are impossible to count. Meaning that a doublet (2 platelets stuck together) may or may not produce more of a fluorescent signal than a triplet or vice versa, depending on how the platelets are stuck together, the distribution of platelet cell surface markers, and how this irregularly shaped clump of platelets enters the aperture. Hence, resolving this smudge of aggregated platelets into a specific number of doublets that should be counted twice, and a region on the scatter plot that only contains 3, 4, 5, and 6, etc., platelets and should be counted 3, 4, 5, or 6 times each hit will be very tough to do. Could some series of experiments using flow cytometry and FACS (a cell sorter that separates out all doublets, for example) be designed that could get there, maybe, but it would be an immense amount of work.
Hence, using flow to answer the question of the presence of aggregates is a good idea, as it will be easy to see a second subpopulation outside the main singlet hits. However, using flow to resolve those aggregate hits back into a platelet count is problematic.
Is There a Solution to this Problem?
Would I advise that the average clinic buy a hemoanalyzer to test their PRP? NO There is too much uncertainty in the results produced. Would I advise that clinics begin putting these counts into orthobiologic registries? NO, for the same reason. There is a caveat to these recommendations below.
Having said all of that, as already discussed, it is still critically important for the field to find a minimum platelet count floor below which using PRP is ineffective. The in-vitro research and early clinical trials on this issue are very compelling. This research should continue with studies that back out the effects of platelet aggregates.
How impacted is the existing research on this topic? Let’s take the recent Patel paper on high-versus low-dose PRP used to treat knee OA (3). How did that paper source the count for the high-dose PRP? They have no information on how they obtained counts, but you can bet it was with a lab-based hemoanalyzer. So, the counts reported in Patel are likely off.
Is there a simple solution here? Is there some way advanced clinics using PRP can guarantee they deliver enough platelets to make a difference? Yes. Here’s what I would do:
- Save your money and don’t buy a hemoanalyzer unless your practice is sophisticated enough to do baseline experiments using your system or technique to back out the effect of aggregates on the count. For example, using a platelet aggregation antagonist (like PGI2 or prostacyclin) and measuring platelets in the PPP to determine the standard error for platelet count across dozens of samples. Or triple checking that deaggregation with flow cytometry.
- Use the highest dose of PRP that is practical. For example, produce a 15X volume reduction PRP with an average platelet capture efficiency of 70% in a patient with a platelet count of 300. It doesn’t take a genius to figure out that if you inject three ccs in a knee, you will be well over any reported platelet floor.
- Flex the concentration based on the known platelet count. If a patient has a low platelet count, flex the concentration of the PRP higher. This means you produce a 20X PRP for that patient’s knee.
The upshot? Like anything in life, there is the simple conception of reality and then the fine print. In this case, the most advanced understanding of what happens when you put PRP into a hemoanalyzer creates significant uncertainty that buying one of these expensive machines is worth it for the average practice.
______________________________________________________________________________
References:
(1) Chemometec. The Ultimate Guide to Cell Counters in the Life Sciences. https://chemometec.com/the-ultimate-guide-to-cell-counters/ Accessed 6/20/24
(2) Berrigan WA, Bailowitz Z, Park A, Reddy A, Liu R, Lansdown D. A Higher Platelet Dose May Yield Better Clinical Outcomes for PRP in the Treatment of Knee Osteoarthritis: A Systematic Review. Arthroscopy. 2024 Mar 19:S0749-8063(24)00206-8. doi: 10.1016/j.arthro.2024.03.018. Epub ahead of print. PMID: 38513880.
(3) Patel S, Gahlaut S, Thami T, Chouhan DK, Jain A, Dhillon MS. Comparison of Conventional Dose Versus Superdose Platelet-Rich Plasma for Knee Osteoarthritis: A Prospective, Triple-Blind, Randomized Clinical Trial. Orthop J Sports Med. 2024 Feb 26;12(2):23259671241227863. doi: 10.1177/23259671241227863. PMID: 38410168; PMCID: PMC10896053.
(4) Bansal, H., Leon, J., Pont, J.L. et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci Rep 11, 3971 (2021). https://doi.org/10.1038/s41598-021-83025-2
(5) Berger DR, Centeno CJ, Steinmetz NJ. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Joint Res. 2019 Feb 2;8(1):32-40. doi: 10.1302/2046-3758.81.BJR-2018-0164.R1. PMID: 30800297; PMCID: PMC6359887.
(6) Anitua E, Sánchez M, Zalduendo MM, de la Fuente M, Prado R, Orive G, Andía I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009 Apr;42(2):162-70. doi: 10.1111/j.1365-2184.2009.00583.x. Epub 2009 Feb 24. PMID: 19250293; PMCID: PMC6496288.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
