Biologics Alliance: The Fine Line Between Evidence and Misinformation
A recent paper published in the journal Arthroscopy makes several now in that journal which are anti-orthobiologics. For this one, I think the authors meant well, but the abstract is so poorly written that it’s getting picked up by news outlets and distorted. Let’s dig in.
Orthopedic Surgery and Orthobiologic Publications
First, if you’re new here, what are orthobiologics? Watch my quick video below to get oriented:
As I have blogged before, there is a strange relationship between most orthopedic surgeons and injections of orthobiologics meant to reduce orthopedic surgery rates. That’s understandable, given that based on our data at Regenexx about 50% or more of elective orthopedic surgeries performed to reduce pain or repair damaged soft tissues like the meniscus, labrum, tendon, or other structures can be replaced for less money and fewer complications by properly performed orthobiologic injections. Hence, that’s a setup for tension between the old guard and the new kid on the block.
The journal Arthroscopy is one that has recently published some poorly written papers that have somehow gotten through peer review. Our group recently rebutted one of them because it made conclusions it couldn’t support (1). Why is this happening? Peer reviewers for journals have biases just like the rest of us. Hence, when a paper comes through that fits with your bias that an injection could never replace the surgeries you perform, you’re probably more likely to greenlight it, even if it’s poorly written or has conclusions that can’t be supported. That’s not an accusation as much as it’s just human nature.
The Problem
This is a headline that recently appeared in an orthopedic surgery focused news outlet:
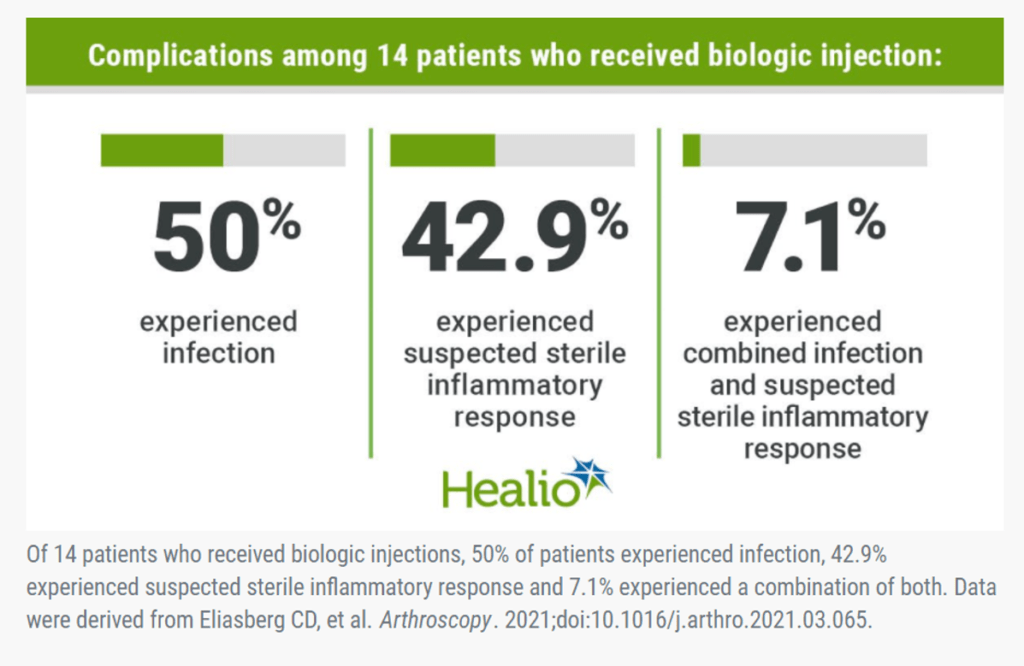
This news story was sent my way by a colleague. The article had an infographic that made statements like:
This claims that of 14 patients treated with orthobiologics, half got infections and the other half had a serious inflammatory response requiring medical care. After tracking the results of tens of thousands of orthobiologic injections in our registry since 2005 and publishing the world’s largest safety paper in orthobiologic injections, this statement is whacked (2). While “whacked” isn’t a medical or scientific team, this statement is so off that it’s the best term to describe it.
What is the actual published infection rate for common orthobiologic injections like bone marrow concentrate from our paper? The infection adverse event rate was 0.3%. That’s consistent with dozens of other papers that have reported the rate of infections and other complications in orthobiologic injections. So where did this crazy statement come from?
The Biologics Association
This nutty statement is from the poorly written abstract of a published paper in the Journal Arthroscopy that was written by members of the Biologics Association (3). I know several physicians involved in this group and all are very level-headed. The group was formed to bring evidence-based medicine into orthobiologics, which is an admirable goal. It includes several organizations I support like IOF and AAPMR. So what’s up?
Even the title of the study is misleading. It is: “Complications following biologic therapeutic injections: a multicenter case series”. The term “case series” means that this is the report of complications of a set number of patients who had a treatment. So what happened here? The actual paper, once you read it, shows that instead of an experience with X number of patients with 50% getting infections or 14 consecutive patients of whom 50% had an infection, this was a questionnaire sent to members of the association asking if they had treated anyone who had a complication. The authors never reported the number of patients who were exposed to orthobiologics nor did they know that number. Meaning the 7 infections could have been the result of thousands of procedures. Hence, this was never a “case series”.
In the end, this was a cherry-picked lowest-level study of the bigger issues around the potential complications of orthobiologics. A good chunk of these cases were due to the Liveyon scandal. So quoting a rate of infection as was done in this new article was inappropriate.
How did this happen? The title and abstract are VERY poorly written and would make one believe that these results were part of some sort of bigger registry study where multiple sites participated. That was likely all the journalist who wrote this story ever read. Hence, hopefully, the Biologics Association will contact the news outlet Helio and others and have them add a correction to the story that the complication rate of an orthobiologic injection doesn’t approach anything close to 50%.
Orthopedic Surgeons in Orthobiologics
The president of the Interventional Orthobiologics Foundation this year is Orthopedic Surgeon Don Bufford, for who I have an immense amount of respect. Like an increasing number of orthopedic surgeons, Don has realized that precise, guided orthobiologic injections can replace a significant chunk of surgical procedures or enhance existing procedures when they need to be done. My hope for the Biologics Association is that they help the field continue to move in the right direction by being very careful what and how they publish. This publication, because of a sloppy abstract, is a net negative and not a net positive for the field. While it’s good to talk about the rare complications of these procedures and we at Regenexx have been publishing those for more than a decade (4-7), producing an abstract that exaggerates those events helps no one and is just more misinformation.
The upshot? I know the leaders of the Biologics Association to be advocates for evidence in this field, which is a good thing. However, this recent paper’s abstract is a mess that needs to be fixed, as most busy doctors and others will only ever read the study abstract and not the full paper. Hopefully, the group will correct that and ask all of the news outlets spreading misinformation to issue clarifications.
_____________________________________________
(1) Centeno C, Burnham R, Rowan P, Le A, Malanga G, Freeman M. Regarding “Intra-articular Mesenchymal Stromal Cell Injections Are No Different From Placebo in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials”. Arthroscopy. 2021 May;37(5):1361-1362. doi: 10.1016/j.arthro.2021.02.026. PMID: 33896482.
(2) Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Aug;40(8):1755-1765. doi: 10.1007/s00264-016-3162-y. Epub 2016 Mar 30. Erratum in: Int Orthop. 2018 Jan;42(1):223. PMID: 27026621.
(3) Eliasberg CD, Nemirov DA, Mandelbaum BR, Pearle AD, Tokish JM, Baria MR, Millett PJ, Shapiro SA, Rodeo SA. Complications following biologic therapeutic injections: a multicenter case series. Arthroscopy. 2021 Apr 16:S0749-8063(21)00331-5. doi: 10.1016/j.arthro.2021.03.065. Epub ahead of print. PMID: 33872744.
(4) Centeno C, Markle J, Dodson E, Stemper I, Williams CJ, Hyzy M, Ichim T, Freeman M. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017 Sep 22;15(1):197. doi: 10.1186/s12967-017-1300-y. PMID: 28938891; PMCID: PMC5610473.
(5) Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, Hanson R. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011 Dec;6(4):368-78. doi: 10.2174/157488811797904371. PMID: 22023622.
(6) Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016 Sep 1;14(1):253. doi: 10.1186/s12967-016-1015-5. PMID: 27585696; PMCID: PMC5009698.
(7) Centeno CJ, Schultz JR, Cheever M, Robinson B, Freeman M, Marasco W. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2010 Mar;5(1):81-93. doi: 10.2174/157488810790442796. PMID: 19951252.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.