Can You Easily Determine MSC Content of BMC at the Bedside?
Dose is a key metric in medicine. However, dosing cells isn’t used in most of orthobiologics. However, is that about to change? Will it be possible to get a dose of stem cells at the bedside when using BMC or Mfat? Let’s dig in.
Dose Matters
Determining the stem cell content of Bone Marrow Concentrate or Mfat at the bedside is one of the holy grails of orthobiologics. Why? Because almost everybody using these technologies has no idea if the dose they’re giving the patient is too little, too much, or just the right amount of stem cells. It’s like the Goldilocks and the three bears story in the Twilight Zone where nobody’s sure which bed is which.
In addition, the existing orthobiologic literature is rife with BMC studies that severely under-dosed patients. That includes the Shapiro et al study and the Anz et al study. Hence, we desperately need to fix this issue so the academics get the memo and design studies using the right dose.
How Has Stem Cell Dose Been Determined for Research?
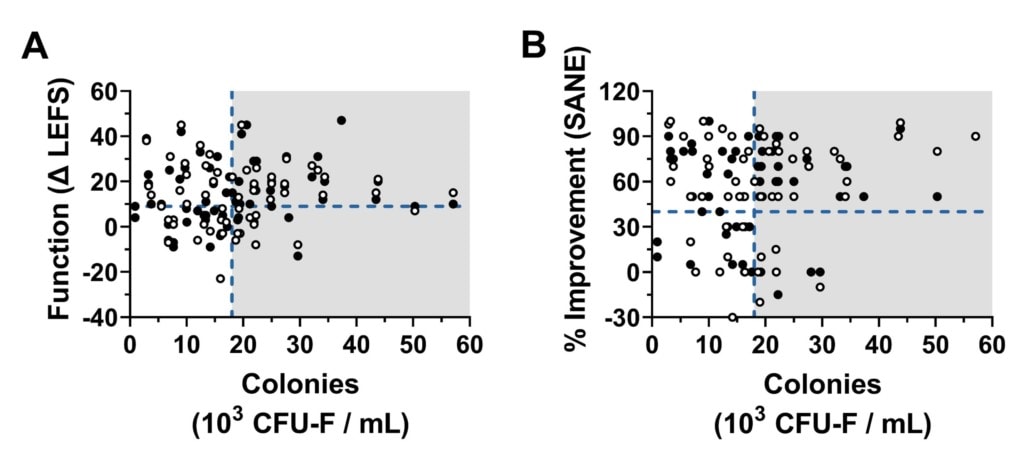
Both Bone Marrow Concentrate (BMC) and micro fragmented fat (Mfat) contain mesenchymal stem cells. The research for BMC shows that you can use a metric called CFU-f and that a higher stem cell content as determined by this metric correlates to outcome (1,2). Meaning the more stem cells in the BMC, the better the results of the treatment. In fact, our team recently had a paper accepted for publication (key diagram above) that adds to that larger dataset showing for the first time that the CFU-f dose correlates with the outcome of using BMC to treat knee arthritis.
CFU-f is pretty easy to use, but it suffers from a serious problem when using it clinically at the bedside. The metric involves plating the BMC into a culture and allowing colonies to form. Each colony represents roughly one mesenchymal stem cell. The problem is that this takes 7-10 days to get a result. Hence, using it at the bedside to determine how many MSCs you’re injecting so you can increase or lower the dose is impossible.
The other problem with CFU-f is that until recently, it could only be used as you treated patients. That meant that every treated patient on any given day had to have a lab employee somewhere close that could plate the BMC in a CFU-f assay. That was a severe waste of resources, so our team published a method that now allows researchers to freeze BMC samples and then run CFU-f assays retrospectively at a later date (3).
Finally, CFU-f has another serious problem. A CFU-f number from one lab CAN NOT be compared to a CFU-f from another lab. I’ve seen people with little lab knowledge try to do this, including companies trying to compare one device to another. The problem is that there are just too many variables like plating density and how the colonies are measured.
Existing Bedside Metrics
There’s only one group to date that has published a metric that can be used at the bedside and is associated with the outcome of treatment. Our group published a paper in 2014 showing that the total cells in the sample (TNCC) are correlated with the outcome of knee arthritis patients (4). The good news is that this metric is easy to measure and our Regenexx network physicians have been using it for years.
Can We Determine Stem Cell Content at the Bedside?
The best possible option would be a way to directly measure stem cell content at the bedside. The only real way to do this using existing technology is flow cytometry. What’s that and how does it work?
Flow cytometry is a technology where cells are stained with a fluorescent dye that attaches to specific cell surface markers. The cells are then lined up using complex fluid dynamics and hit with a LASER at a specific wavelength which causes them to fluoresce. That fluorescence is measured to determine if a specific cell surface marker is present.
The problem is that the classic definition of what is or isn’t an MSC has been complex. The ISCT definition has been (5):
- Has these markers: CD105, CD73, and CD90
- Doesn’t have these markers: CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR
Pretty complex, huh?
However, we’ve been watching a new single marker theory to identify MSCs. That’s been around for about a decade and now there is more and more research published confirming that this approach works (6-8).
CD271
Cell surface markers have a “CD” in front of them which stands for “Cluster of Differentiation”. CD271 is interesting as it’s present on mesenchymal stem cells in bone marrow and adipose tissue and can be used to sort them (this doesn’t work with umbilical cord tissue). MSCs isolated with this marker are more chondrogenic and osteogenic (better at repairing cartilage and bone).
Is This Real and Does It Correlate to CFU-f?
Good scientists never take the word of someone else, especially when it’s an emerging scientific factoid like this one. Hence, our lab team has been hard at work trying to correlate CD271 with our CFU-f measurements.
Here’s one of our lab scientists, Dustin Berger using our newest flow cytometer measuring CD271:

It should be noted that this flow cytometer is specially designed to measure rare events like MSCs. Here Dustin has gated out the events he doesn’t want and is measuring the CD271 hits in this bone marrow sample.
Below are CFU-f assay plates on the same samples where each dot represents a stem cell colony:
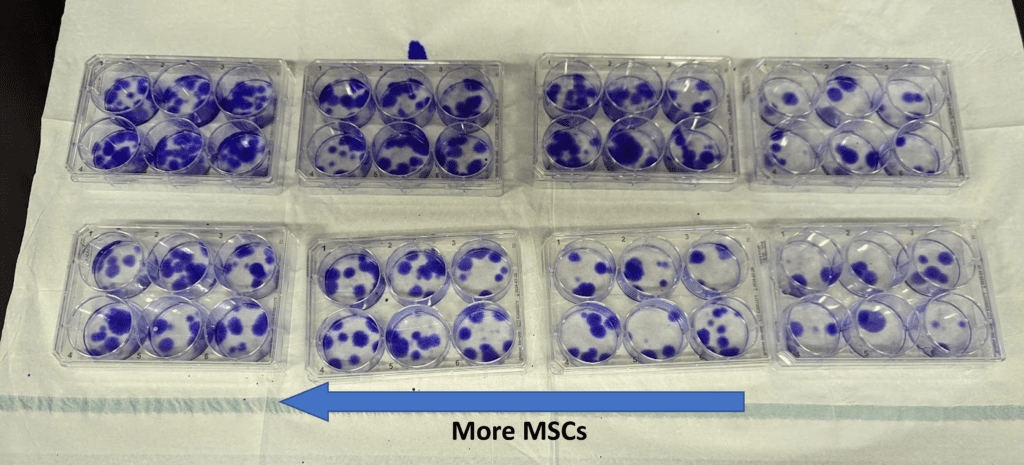
So far so good. The CD271 data seems to match up with the CFU-f numbers. We have more data to collect, so this is not yet fully confirmed, but looks promising.
Could Providers Do This at a Clinic?
I’ve seen sales reps selling simple bedside flow cytometry devices. When we’ve tested these against more sophisticated and expensive units like the ones we use in our research lab, thus far we’ve been disappointed with the results. The problem is the “gating” that needs to be done to filter out junk signals from the ones you want.
In addition, there’s the “rare event” issue. The flow cytometer above uses “acoustic focusing” to allow better detection of the occasional MSC in a bone marrow or adipose sample. This technology is not yet in common use in cheaper flow machines.
Finally, you basically need a Ph.D. level lab team member to run and adjust these machines in real-time. Hence, while a few practices around the country involved in orthobiologics like ours and maybe a handful of others with big budgets would be able to do this, it’s very unlikely that the average practice that uses orthobiologics could pull this off.
Could we see special devices or assays that use CD271? That’s possible, but much would depend on the regulatory lab test costs to determine if this is financially feasible. If it is, those simple bedside readings may markedly improve BMC response rates.
The upshot? The good news is that there now seems to be a reliable way to quickly measure the stem cell content of a BMC injection. The bad news is that this technology may be confined for now to big orthobiologics practices with serious research budgets. However, maybe someone brilliant will bring an easy-to-use CD271 count to the bedside? Time will tell.
_________________________________________
References:
(1) Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015 Jan;33(1):146-56. doi: 10.1002/stem.1845. PMID: 25187512.
(2) Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002 Dec;(405):14-23. doi: 10.1097/00003086-200212000-00003. PMID: 12461352.
(3) Berger DR, Aune ET, Centeno CJ, Steinmetz NJ. Cryopreserved bone marrow aspirate concentrate as a cell source for the colony-forming unit fibroblast assay. Cytotherapy. 2020 Sep;22(9):486-493. doi: 10.1016/j.jcyt.2020.04.091. Epub 2020 Jun 19. PMID: 32565131.
(4) Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015 Sep 18;16:258. doi: 10.1186/s12891-015-0714-z. PMID: 26385099; PMCID: PMC4575428.
(5) Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. doi: 10.1080/14653240600855905. PMID: 16923606.
(6) Álvarez-Viejo M, Menéndez-Menéndez Y, Otero-Hernández J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J Stem Cells. 2015 Mar 26;7(2):470-6. doi: 10.4252/wjsc.v7.i2.470. PMID: 25815130; PMCID: PMC4369502.
(7) Watson JT, Foo T, Wu J, Moed BR, Thorpe M, Schon L, Zhang Z. CD271 as a marker for mesenchymal stem cells in bone marrow versus umbilical cord blood. Cells Tissues Organs. 2013;197(6):496-504. doi: 10.1159/000348794. Epub 2013 May 14. PMID: 23689142.
(8) Kohli, N., Al-Delfi, I.R.T., Snow, M. et al. CD271-selected mesenchymal stem cells from adipose tissue enhance cartilage repair and are less angiogenic than plastic adherent mesenchymal stem cells. Sci Rep 9, 3194 (2019). https://doi.org/10.1038/s41598-019-39715-z

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
