Digging the Hole Deeper and Deeper: Biolabs Fluid Flow
One thing I learned many years ago as a young doctor was that you don’t mess with Medicare billing. It’s literally like kryptonite to every medical practice or hospital. No matter how big you are, if you screw up or worse yet, purposefully bill Medicare for things that you shouldn’t, the feds will take you down hard. That’s why it surprises me that Biolabs and Fluid Flow are doubling and tripling down on their concept that you can bill Medicare for their amniotic product. Let’s dig in.
Medicare Is a Tough Mistress
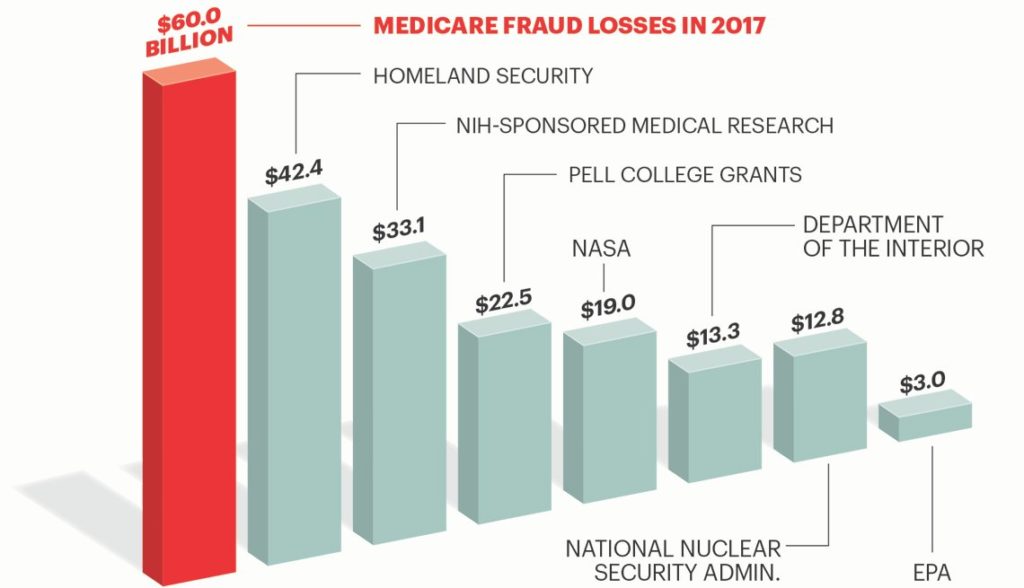
The feds lose big bucks in fraud and abuse through Medicare. Hence, they are VERY serious about getting money back from Medicare claims that have been paid in error. Add in contractors who get paid a chunk of every dollar they recover and you have a perfect storm that every physician never wants to experience. Meaning, once a Medicare contractor dives deep into billing practices that may be shaky, if there is anything to find that will result in a payback of funds to Medicare, they WILL find it.
Amniotic Fluid Coverage and Q-codes
I’ve covered this a number of times already, so if you want to get up to snuff, watch my video:
Changing Marketing Stories
When I first began investigating Fluid Flow and BioLab, the story was that they had the Q-code and that the Medicare LCD was just slow to catch up. An “LCD” is the medical coverage guidelines that states what Medicare will and won’t cover. IMHO, now that the company knows that there will be no Medicare LCDs in the near future that will authorize the use of Fluid Flow for MSK conditions, they need a change of marketing strategy. Hence, now it’s changed to try to convince doctors that LCDs no longer matter much.
That happened recently when Biolab put on a seminar that tried to convince providers that the 21st Century Cure Act had deemphasized the importance of LCDs and instead the reimbursement focus has changed to “Articles”. When I called a Medicare Fraud and Abuse expert I was told that none of this was actually true. However, that hasn’t stopped Fluid Flow from going deeper and deeper down this rabbit hole.
The Biolabs Twostep
First, after using the expression “two-step” my entire life, I actually didn’t know this was a real dance popular in Texas. That’s despite completing my residency at Baylor in Houston. I guess I didn’t get out much.
Second, Biolabs has tripled down on the idea that Medicare covers their product for things like knees and backs. One of the ways that they’ve done this is to have a podiatrist who states that he’s a professional coder dive deeper into the idea that an HCPCS code rubber-stamping session is more credible to determine coverage than a Medicare LCD. I call this the BioLabs two-step.
A Podiatrist Who is a Certified Coder?
One of the stranger videos I have seen in the ongoing saga of claimed Medicare reimbursement for amniotic fluid used to treat orthopedic problems is one created by a Podiatrist who states he’s a certified coder. Let’s analyze what’s being said.
The is a podiatrist named Jeff Lehrman. He gave a webinar for BioLabs supporting their idea of Medicare reimbursement for orthopedic indications. If anyone has any info on how Dr. Lehrman is connected to BioLabs, please don’t hesitate to write me at [email protected]. This is from his webinar:

This statement that Medicare billing information should come from CMS is reasonable. However, then Jeff, like the last BioLabs video, IMHO, goes off the rails. He begins talking about his experience in attending Medicare HCPCS meetings where Q codes are granted. He then states:
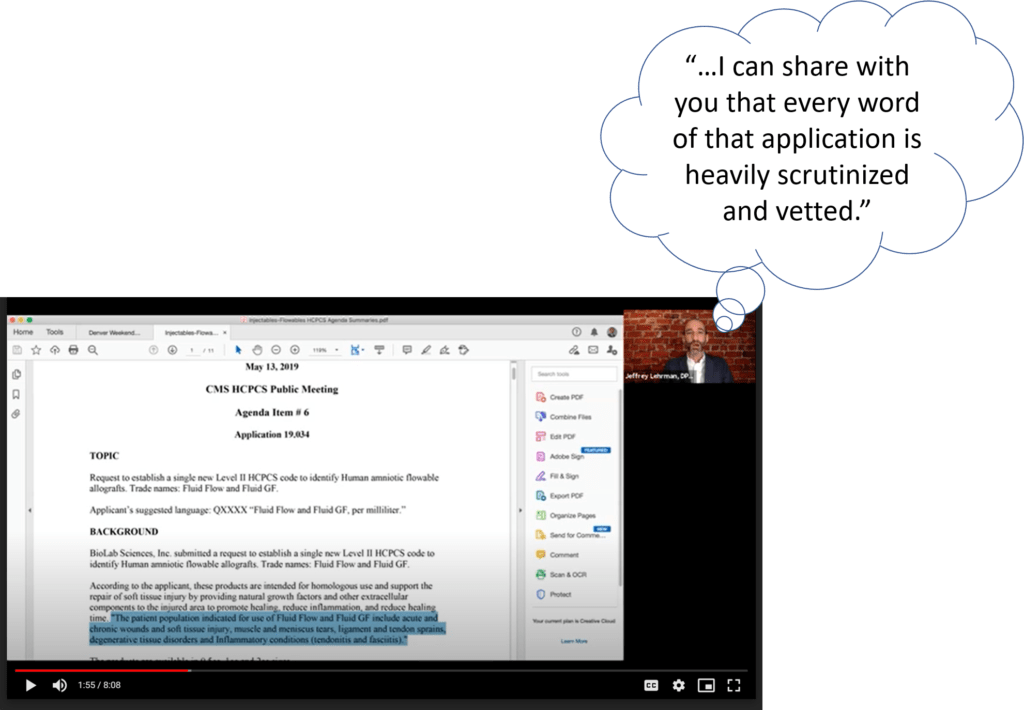
So let’s review what’s being said here, as this is the major crux of Jeff Lehrman’s presentation and BioLabs thesis of why it’s OK to bill Medicare for their Fluid Flow product to treat things like knee arthritis and back pain. BioLab claims that since they submitted an application to Medicare that their amniotic product can be used to treat certain things and that this was rubber-stamped in a Q-code granting session, then Medicare MUST pay for its use in those indications. So what did that application say? Here is the wording:
“The patient population indicated for use of Fluid Flow and Fluid GF include acute and chronic wounds and soft tissue injury, muscle and meniscus tears, ligament and tendon sprains, degenerative tissue disorders and inflammatory conditions (tendonitis and fasciitis)”
So what part of this application was “heavily scrutinized and vetted”? Let’s vet that vetting by looking at two critical categories:
- Regulatory
- Clinical Evidence
Let’s dive into each.
Regulatory
Was the CMS panel that “heavily scrutinized and vetted” this application looking at regulatory issues? BioLab Fluid Flow has a simple, 45-minute 361 tissue registration. Intended use clinical indications like the ones above are not permitted by FDA for this product type. In fact, by BioLabs putting in writing that FluidFlow is intended to be used to treat something like meniscus tears, that assertion changes the regulatory status of this product. IMHO it takes it from a registered tissue to an unapproved drug product that would take years of clinical trials to be able to make that claim. BioLabs has included almost a dozen indication claims in this application and each one requires extensive FDA-approved trials to support. Hence, we can rest assured that the CMS panel that approved this application never “heavily scrutinized and vetted” the regulatory issues here.
Clinical Evidence
Maybe there was a significant body of peer-reviewed and published clinical evidence that BioLabs presented that was “heavily scrutinized and vetted”? I ran several searches at the US National Library of Medicine on published studies on the Fluid Flow product. All of them came back like this:
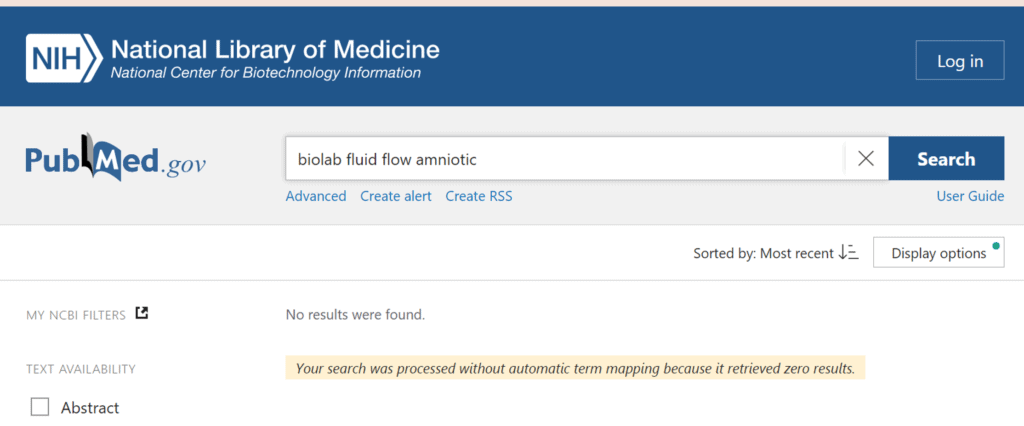
Meaning that there is NO published clinical data that I could find that shows that Fluid Flow is clinically effective in ANY of the claimed medical indications. So was the clinical research on this product “heavily scrutinized and vetted”? NOPE.
So what was “heavily scrutinized and vetted”? Spelling? Syntax? Certainly not anything that would matter to CMS in approving coverage for almost a dozen new clinical indications for Amniotic Fluid.
You Just Can’t Make This Stuff Up
In doing research for this blog I chanced on this:
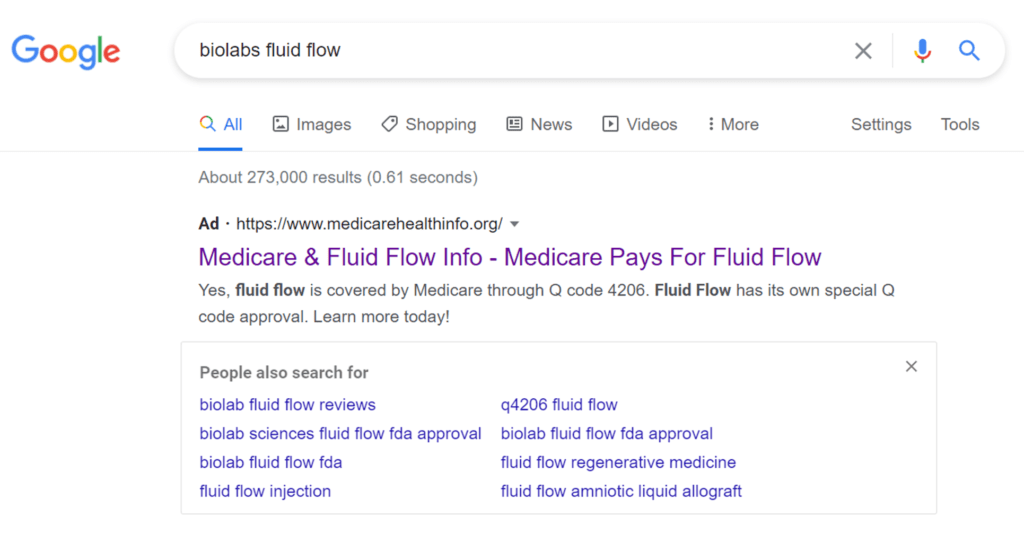
This is a Google ad that says, “Medicare Pays for Fluid Flow”. This ad says that the group providing the information is www.medicarehealthinfo.org. That sure sounds like it’s a non-profit because of the .org domain. However, the group apart from the Fluid Flow ad, near as I can tell, doesn’t exist. This is what greets you when you click:
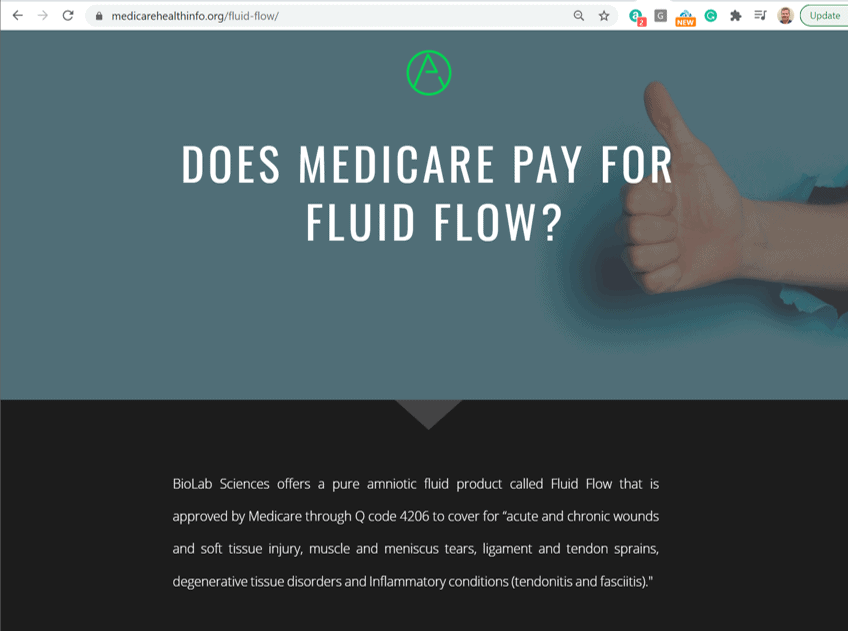
This website basically repeats the BioLab sales pitch that Medicare covers this stuff for the indications it got rubber-stamped for at a Medicare code granting session. Who put this up? I know I’ve seen that “A” logo at the top before. This looks similar:

That’s one of the billing companies that works with BioLab. So is the “A” logo in the ad a rework of the Total Ancillary logo? Not sure, but it sure looks similar.
Suffice it to say that this website does not represent a non-profit nor Medicare. However, it is created to look like it’s an official statement that Medicare “covers” this product for orthopedic problems.
The upshot? You gotta love the BioLabs commitment to this marketing spiel. The word on the street is that we will soon find out if they’re right about this concept. In the meantime, if I were a betting man, I wouldn’t bet any of my hard-earned money that BioLabs fluid flow is actually covered by Medicare to treat knee or back pain. In fact, I’ll take a sizable bet that huge and devasting clawbacks on the way for providers uneducated enough to believe this song and dance.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
